| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
Purity: =99.23%
| Targets |
Natural alkaloid from seeds of PEGANUM
|
|---|---|
| ln Vitro |
Harmaline and its derivatives were identified as anti-MDR agents against various highly resistant and Pakistani MDR clinical isolates of E. coli. These compounds may serve as the leads for further studies towards the development of treatment against the infections caused by MDR E. coli.[1]
Experiments were conducted on Control (Salin) and Experiment (Harmaline) groups, generating a dataset for developing predictive models. Because the dataset has a limited number of samples, we utilized models that are effective with small datasets. Among different groups of regression models (linear, ensemble, and tree models), the ensemble models, specifically the LGB method, can achieve better performance. The results demonstrate accurate prediction of first spike latency, with an average mean squared error of 0.0002 and mean absolute error of 0.01 in 10-fold cross-validation. The research suggests the potential of machine learning in forecasting the first spike latency, allowing reliable estimation without the need for extensive animal testing. This intelligent predictive system facilitates efficient analysis of first spike latency changes in both healthy and unhealthy brain cells, streamlining experimentation and providing more detailed insights into the captured signals.[2] |
| ln Vivo |
Depression is a mental disorder characterised by persistent low mood, anhedonia and cognitive impairment that affects an estimated 3.8% of the world's population, including 5% of adults. Peganum harmala L. (P. harmala) is a medicinal plant and has been reported to be effective against Alzheimer's disease, Parkinson's disease and depression. The present study was aimed to evaluate the behavioral and pharmacological effects of P. harmala seed extract in rats exposed to chronic unpredictable mild stress (CUMS) in vivo and to investigate the mechanism of action. CUMS-exposed rats were treated with P. harmala extract (75 and 150 mg/kg, i.p.) for 2 weeks. HPLC analysis was used to determine the concentration of Harmaline and harmine alkaloids in the extract. Heavy metal analysis in seeds was performed by ICP-MS. Our results showed that P. harmala at the dose of 150 mg/kg significantly reduced the depressive-like behaviors in CUMS-exposed rats, as evidenced by increased sucrose consumption in the sucrose preference test (SPT), decreased immobility time in the forced swim test (FST) and plasma corticosterone levels, increased the time spent in open arms in the elevated plus maze (EPM), and improved memory and learning in the passive avoidance test (PAT). In addition, P. harmala decreased monoamine oxidase-A (MAO-A) levels, and increased serotonin (5-HT), dopamine (DA), and noradrenaline (NA) levels in the brains of rats exposed to CUMS. P. harmala decreased the expression of the pro-inflammatory transcription factor nuclear factor-κB (NF-κB), and increased the antioxidant nuclear factor erythroid 2-related factor 2 (Nrf2) in rat brain. Furthermore, P. harmala improved brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) protein expression in rat brain. In conclusion, P. harmala at a dose of 150 mg/kg is more effective in preventing depressive-like behavior in CUMS-exposed rats by improving neurotransmitter levels, reducing oxidative stress, suppressing neuroinflammation and activating the BDNF/TrkB pathway, all of which are important in the pathogenesis of depression.[2]
|
| Cell Assay |
Multidrug resistance (MDR) is a major challenge in the treatment of infectious diseases. The MDR in urinary tract infection causing bacteria, such as Escherichia coli, has made treatment of UTI very difficult.
Objective: The aims of the current study were to synthesize a library of harmaline derivatives, and to evaluate their activity against various strains of multi-drug resistance (MDR) E. coli.
Method: Harmaline derivatives were synthesized by the reaction of harmaline (1) with various acid halides and anhydrides. These compounds were subjected to susceptibility determination by in vitro MTT assay. The changes in morphology of the bacterial cells after the treatment with harmaline (1) and its new derivatives 2 and 3 were studied through scanning electron, atomic force and fluorescence microscopy. Effect of harmaline and its derivatives on the production of Reactive Oxygen Species (ROS) in MDR E. coli was assessed through lucigenin chemiluminescence assays.
Results: The selected compounds assisted the fluorescently labeled dye DiBAC4(3) to bind to the lipid rich intra-cellular entities, and thus produced a sharp green fluorescence by easily penetrating into the compound-induced depolarized membrane of MDR E. coli. These compounds have also triggered a significant generation of ROS from bacterial cells as compared to the conventional antibiotics. The current study demonstrated that harmaline (1), and its derivatives 2 and 3 were identified as anti-MDR agents against MDR strains of E. coli. Antibacterial effect of compounds 1-3 on MDR E. coli is possibly due to membrane depolarization due to ROS-induced damage to the bacterial cell membrane.[1]
|
| References |
[1]. Harmaline and its Derivatives Against the Infectious Multi-Drug Resistant Escherichia coli. Med Chem. 2017;13(5):465-476.
[2]. Peganum harmala L. seed extract attenuates anxiety and depression in rats by reducing neuroinflammation and restoring the BDNF/TrkB signaling pathway and monoamines after exposure to chronic unpredictable mild stress. Metab Brain Dis . 2024 Aug 22. doi: 10.1007/s11011-024-01416-6. [3]. Comparison of Regression Methods to Predict the First Spike Latency in Response to an External Stimulus in Intracellular Recordings for Cerebellar Cells. Stud Health Technol Inform . 2024 Aug 22:316:796-800. |
| Additional Infomation |
Background: The present study aimed to elucidate the potential anticancer activity and mechanism of P. harmala's alkaloid extract, harmine (HAR), and harmaline (HAL) in HCT-116 colorectal cancer cells.
Methods and results: P. harmala's alkaloid was extracted from harmala seeds. HCT-116 cells were treated with P. harmala's alkaloid extract, HAR and HAL. Cytotoxicity was determined by MTT assay, apoptotic activity detected via flow cytometry and acridine orange (AO)/ethidium bromide (EB) dual staining, and cell cycle distribution analyzed with flow cytometry. The mRNA expression of Bcl-2-associated X protein (Bax) and glycogen synthase kinase-3 beta (GSK3β) was measured by real-time PCR. Furthermore, the expression of Bax, Bcl-2, GSK3β and p53 proteins, were determined by western blotting. The findings indicated that, P. harmala's alkaloids extract, HAR and HAL were significantly cytotoxic toward HCT116 cells after 24 and 48 h of treatment. We showed that P. harmala's alkaloid extract induce apoptosis and cell cycle arrest at G2 phase in the HCT116 cell line. Downregulation of GSK3β and Bcl-2 and upregulation of Bax and p53 were observed.
Conclusion: The findings of this study indicate that the P. harmala's alkaloid extract has anticancer activity and may be further investigated to develop future anticancer chemotherapeutic agents.Mol Biol Rep. 2024 Jun 13;51(1):732. doi: 10.1007/s11033-024-09655-7. https://pubmed.ncbi.nlm.nih.gov/38872006/
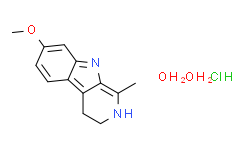
Harmaline is a harmala alkaloid in which the harman skeleton is methoxy-substituted at C-7 and has been reduced across the 3,4 bond. It has a role as a oneirogen. It derives from a hydride of a harman. Harmaline has been reported in Passiflora phoenicia, Daphnia pulex, and other organisms with data available. LOTUS - the natural products occurrence database A beta-carboline alkaloid isolated from seeds of PEGANUM. View More
Therapeutic Uses Absorption, Distribution and Excretion Harmaline, a known type A monoamine oxidase (MAO) inhibitor in adult brain of various species was found to elevate whole brain levels of dopamine and serotonin (5-HT) in rat fetuses of mothers injected 2-4 hr before Caesarean delivery. Similar stimulatory effects were observed for the norepinephrine metabolite 3-methoxy-4-hydroxy-phenylglycol (MHPG), however, no significant effect was obtained for norepinephrine. The dopamine metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC) and the 5-HT metabolite 5-hydroxyindole acetic acid (5-HIAA) were decreased with the same treatment. These results imply that harmaline or one of its metabolites may cross the placental barrier to affect the fetal brain system not merely as a type A MAO inhibitor (i.e., relatively 5-HT-specific), but possibly also as a stimulatory agent for aldehyde reductase or catechol-O-methyltransferase (COMT) or alternately as an agent inhibiting the conjugation, efflux, or turnover of biogenic amine metabolites such as MHPG. PMID:2465555 Okonmah AD et al; Pharmacology 37 (3): 203-8 (1988) Metabolism / Metabolites The psychotropic beta-carboline alkaloids, showing high affinity for 5-hydroxytryptamine, dopamine, benzodiazepine, and imidazoline receptors and the stimulation of locus coeruleus neurons, are formed endogenously from tryptophan-derived indolealkylamines through the Pictet-Spengler condensation with aldehydes in both plants and mammals. Cytochromes P450 1A1 (18.5), 1A2 (20), and 2D6 (100) catalyzed the O-demethylation of harmaline, and CYP1A1 (98.5), CYP1A2 (35), CYP2C9 (16), CYP2C19 (30), and CYP2D6 (115) catalyzed that of harmine (relative activities). The dehydrogenation/aromatization of harmaline to harmine was not carried out by aromatase (CYP19), CYP1A2, CYP2C9, CYP2D6, CYP3A4, pooled recombinant cytochromes P450, or human liver microsomes (HLMs). Kinetic parameters were calculated for the O-demethylations mediated by each isozyme and by pooled HLMs. K(cat) (min(-1)) and Ku (uM) values for harmaline were: CYP1A1, 10.8 and 11.8; CYP1A2, 12.3 and 13.3; CYP2C9, 5.3 and 175; CYP2C19, 10.3 and 160; and CYP2D6, 39.9 and 1.4. Values for harmine were: CYP1A1, 45.2 and 52.2; CYP1A2, 9.2 and 14.7; CYP2C9, 11.9 and 117; CYP2C19, 21.4 and 121; and CYP2D6, 29.7 and 7.4. Inhibition studies using monoclonal antibodies confirmed that CYP1A2 and CYP2D6 were the major isozymes contributing to both harmaline (20% and 50%, respectively) and harmine (20% and 30%) O-demethylations in pooled HLMs. The turnover numbers for CYP2D6 are among the highest ever reported for a CYP2D6 substrate. Finally, CYP2D6-transgenic mice were found to have increased harmaline and harmine O-demethylase activities as compared with wild-type mice. These findings suggest a role for polymorphic CYP2D6 in the pharmacology and toxicology of harmine and harmaline. PMID:12649384 Yu AM et al; J Pharmacol Exp Ther 305 (1): 315-22 (2003) Mechanism of Action Three psychological active principles from the seeds of Peganum harmala L., harmine, harmaline and harmalol, showed vasorelaxant activities in isolated rat thoracic aorta preparations precontracted by phenylephrine or KCl with rank order of relaxation potency of harmine > harmaline > harmalol. The vasorelaxant effects of harmine and harmaline (but not harmalol) were attenuated by endothelium removal or pretreatment with a nitric oxide (NO) synthase Nomega-nitro-L-arginine methyl ester. In cultured rat aortic endothelial cells, harmine and harmaline (but not harmalol) increased NO release, which was dependent on the presence of external Ca2+. In endothelium-denuded preparations, pretreatment of harmine, harmaline or harmalol (3-30 microM) inhibited phenylephrine-induced contractions in a non-competitive manner. Receptor binding assays indicated that all 3 compounds interacted with cardiac alpha1-adrenoceptors with comparable affinities (Ki value around 31 - 36 microM), but only harmine weakly interacted with the cardiac 1,4-dihydropyridine binding site of L-type Ca2+ channels (Ki value of 408 microM). Therefore, the present results suggested that the vasorelaxant effects of harmine and harmaline are attributed to their actions on the endothelial cells to release NO and on the vascular smooth muscles to inhibit the contractions induced by the activation of receptor-linked and voltage-dependent Ca2+ channels. The vasorelaxant effect of harmalol was not endothelium-dependent. PMID:11325023 Shi CC et al; Jpn J Pharmacol 85 (3): 299-305 (2001) |
| Molecular Formula |
C13H19CLN2O3
|
|---|---|
| Molecular Weight |
286.7546
|
| Exact Mass |
286.108
|
| CAS # |
6027-98-1
|
| PubChem CID |
2723643
|
| Appearance |
Typically exists as solid at room temperature
|
| Boiling Point |
426.4ºC at 760 mmHg
|
| Melting Point |
232-240 °C
|
| Flash Point |
211.7ºC
|
| Source |
PEGANUM
|
| LogP |
2.65
|
| Hydrogen Bond Donor Count |
4
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
1
|
| Heavy Atom Count |
19
|
| Complexity |
302
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
Cl[H].O(C([H])([H])[H])C1C([H])=C([H])C2=C(C=1[H])N([H])C1C(C([H])([H])[H])=NC([H])([H])C([H])([H])C=12.O([H])[H].O([H])[H]
|
| InChi Key |
LCEKUHFBUFUSSY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H14N2O.ClH.2H2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13;;;/h3-4,7,15H,5-6H2,1-2H3;1H;2*1H2
|
| Chemical Name |
7-methoxy-1-methyl-4,9-dihydro-3H-pyrido[3,4-b]indole;dihydrate;hydrochloride
|
| Synonyms |
Harmaline hydrochloride dihydrate; UNII-5B4DGH2M9R; 6027-98-1; 63885-08-5; 5B4DGH2M9R; 7-methoxy-1-methyl-4,9-dihydro-3H-pyrido[3,4-b]indole;dihydrate;hydrochloride; MFCD00150052; 7-Methoxy-1-methyl-4,9-dihydro-3H-pyrido[3,4-b]indole hydrochloride dihydrate;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4874 mL | 17.4368 mL | 34.8736 mL | |
| 5 mM | 0.6975 mL | 3.4874 mL | 6.9747 mL | |
| 10 mM | 0.3487 mL | 1.7437 mL | 3.4874 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.