
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Sotorasib (AMG-510; AMG510; Lumakras; Lumykras) is a novel, first-in-class and covalent/irreversible inhibitor of KRAS G12C that has been approved by FDA on 5/28/2021 to treat non-small-cell lung cancer (NSCLC). It specifically targets the most common mutation among the three subtypes of Ras proteins (KRas, NRas, and HRas), the KRAS G12C mutation. KRAS gene mutations (e.g. G12C, G12V, G12D, and G13D) are present in approximately 30% of human cancers, and are most common in pancreatic cancer, lung adenocarcinoma, colorectal cancer, gall bladder cancer, thyroid cancer, and bile duct cancer. About 25% of NSCLC patients also have KRAS mutations, and some research suggests that these mutations are associated with a poorer prognosis for NSCLC patients. It has recently been discovered that V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations confer resistance to epidermal growth factor receptor (EGFR) targeted therapies in colorectal cancer; as such, knowledge of the KRAS mutational status can be crucial before TKI therapy is prescribed. All things considered, patients diagnosed with lung adenocarcinoma, colorectal cancer, or pancreatic cancer require novel medical interventions; this is particularly true for those whose cancers are marked by a KRAS mutation, as well as those who have advanced following chemotherapy.
| Targets |
KRAS(G12C)
|
|---|---|
| ln Vitro |
AMG 510 had little effect on KRAS (C118A) but inhibits the nucleotide exchange of recombinant mutant KRAS (G12C/C118A) when catalyzed by SOS1. Additionally, AMG 510 specifically reduces the viability of KRAS p.G12C mutant lines while having no effect on cell lines harboring other KRAS mutations[1].
|
| ln Vivo |
AMG 510 quickly and irreversibly binds to KRAS (G12C) in preclinical tumor models, resulting in long-lasting inhibition of the mitogen-activated protein kinase (MAPK) signaling pathway. When administered orally (once daily) as a single agent, AMG 510 has the ability to induce tumor regression in KRASG12C cancer-bearing mice models[2].
|
| Enzyme Assay |
Activating mutations in RAS represent the most common oncogenic driver mutation in cancer. The single amino acid substitution of cysteine for glycine at position 12 (KRASG12C) is frequently found in solid malignancies, particularly in lung adenocarcinoma (~13%), colorectal adenocarcinoma (3%), and pancreatic adenocarcinoma (~1%). Recently it has been demonstrated that KRASG12C can be targeted with covalent small molecule inhibitors which react with the mutant cysteine adjacent to the switch II pocket (SIIP), locking KRAS in its inactive GDP-bound state. We describe here the discovery and in vitro characterization of AMG 510, a covalent inhibitor of KRASG12C possessing potent biochemical and cellular activity, as well as robust in vivo efficacy. AMG 510 inhibited SOS1-catalyzed nucleotide exchange of recombinant mutant KRASG12C/C118A but had minimal effect on KRASC118A, which is wildtype at position 12. The observed rate constant (kinact/Ki) of covalent modification of KRASG12C by AMG 510 was determined biochemically by mass spectrometry as well as in the cellular context (kobs/[I]). Cysteine proteome analysis of cells treated with AMG 510 revealed that only the G12C-containing peptide of KRAS was covalently modified. AMG 510 inhibited KRAS signaling as measured by ERK phosphorylation in all KRAS p.G12C cell lines tested, but did not inhibit phosphorylation of ERK in cell lines lacking the KRAS p.G12C mutation. Cellular occupancy of KRASG12C by AMG 510 was determined by mass spectrometry and correlated well with inhibition of ERK phosphorylation. AMG 510 also selectively impaired the viability of KRAS p.G12C mutant lines. Combination treatment of AMG 510 with inhibitors of other cellular signaling pathways exhibited evidence for synergistic effects on cell viability. Treatment of KRAS p.G12C lines with covalent KRASG12C inhibitors increased the expression of HLA. To test the impact of KRASG12C inhibition on immune surveillance in vivo, we generated a syngeneic tumor cell line that is suitable for testing AMG 510 in combination with checkpoint inhibitor therapies and characterized this line in vitro. AMG 510 is currently being evaluated in a Phase I study in patients with solid tumors harboring KRAS p.G12Cmutations[1].
|
| Cell Assay |
Cell Line: NCI-H358 and MIA PaCa-2 cells
Concentration: 1-10 μM Incubation Time: 72 hours Result: Potently impaired cellular viability in both NCI-H358 and MIA PaCa-2 (IC50≈0.006 μM and 0.009 μM respectively). Somatic activating mutations of RAS family members are tumor driver mutations found in an estimated 21% of all cancers. Oncogenic KRAS mutations at residues G12, G13, and Q61 represent the most common RAS mutations found in solid malignancies. The prevalence of KRAS p.G12C tumors is ~13% of lung adenocarcinoma (including NSCLC), 3% of colorectal carcinoma (CRC), and 1% to 2% of numerous other solid tumors, representing an unmet medical need. We have developed AMG 510, an orally bioavailable, covalent inhibitor of KRASG12C with potent biochemical and cellular activity, and robust in vivo efficacy. AMG 510 inhibited SOS-catalyzed nucleotide exchange of recombinant mutant KRASG12C/C118A but had minimal effect on KRASC118A, which is wildtype at position 12. In cellular assays, AMG 510 covalently modified KRASG12C and inhibited KRASG12C signaling as measured by phosphorylation of ERK1/2 (p-ERK) in all KRAS p.G12C-mutant cell lines tested but did not inhibit p-ERK in cell lines with various other KRAS mutations. AMG 510 also selectively impaired viability of KRAS p.G12C mutant cell lines but did not affect cell lines with other KRAS mutations[5]. |
| Animal Protocol |
Female ICR-SCID mice
100 mg/kg o.g. The RAS gene family encodes the small GTPase proteins NRAS, HRAS, and KRAS, which play an essential role in cellular growth and proliferation. KRAS is one of the most frequently mutated oncogenes in human cancer, with KRAS p.G12D, p.G12V, and p.G12C constituting the major mutational subtypes across lung, colon, and pancreatic cancers. Despite more than three decades of research, indirect approaches targeting KRAS mutant cancers have largely failed to show clinical benefit, and direct approaches have been stymied by the apparently ‘undruggable’ nature of KRAS. Cysteine-12 of KRASG12C has recently emerged as a unique vulnerability in KRAS-mutant cancers, and a small number of cysteine-reactive inhibitory tool molecules have been disclosed. We here report independent efforts to identify cysteine-reactive molecules capable of selectively inhibiting KRASG12C. Through iterative screening and structural biology efforts, we identified a novel Cys12-reactive inhibitor scaffold that derived its potency from occupancy of a previously unknown cryptic pocket induced by side-chain motion of the His95 residue of KRAS. Employing a scaffold-hopping approach, we leveraged knowledge of this cryptic pocket to design a series of N-aryl quinazolin-2(1H)-one-based inhibitors that demonstrated significantly enhanced potency relative to prior tool compounds. Extensive optimization of these leads led to the identification of a highly potent, selective, and well-tolerated inhibitor of KRASG12C, which was nominated for clinical development as AMG 510. In preclinical tumor models, AMG 510 rapidly and irreversibly binds to KRASG12C, providing durable suppression of the mitogen-activated protein kinase (MAPK) signaling pathway. Dosed orally (once daily) as a single agent, AMG 510 is capable of inducing tumor regression in mouse models of KRASG12C cancer. AMG 510 is, to the best of our knowledge, the first direct KRASG12C therapeutic to reach human clinical testing and is currently in a Phase I clinical trial evaluating safety, tolerability, PK, and efficacy in subjects with solid tumors bearing the KRAS p.G12C mutation (NCT03600883). |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
A 960 mg once daily dose of sotorasib reaches a Cmax of 7.50 µg/mL, with a median Tmax of 2.0 hours, and an AUC0-24h of 65.3 h\*µg/mL. Sotorasib is 74% eliminated in the feces and 6% eliminated in the urine. 53% of the dose recovered in the feces and 1% of the dose recovered in the urine is in the form of the unchanged parent compound. The volume of distribution of sotorasib is 211 L. Sotorasib has an apparent clearance at steady state of 26.2 L/h. Metabolism / Metabolites Sotorasib is predominantly metabolized through conjugation or by CYP3As. Biological Half-Life Sotorasib has a terminal elimination half life of 5.5 ± 1.8 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In the prelicensure clinical trials of sotorasib in patients with solid tumors harboring KRAS G12C mutations, liver test abnormalities were frequent although usually self-limited and mild. Some degree of ALT elevations arose in 38% of sotorasib treated patients and were above 5 times the upper limit of normal (ULN) in 6% to 7%. In these trials that enrolled approximately 427 patients, sotorasib was discontinued early due to increased AST or ALT in 8% of patients. In addition, a small proportion of patients developed significant hepatotoxicity requiring sotorasib discontinuation and treatment with corticosteroids. The liver test abnormalities had a median onset of 9 weeks after initiation of therapy. While serum aminotransferase elevations were occasionally quite high (5 to 20 times upper limit of normal), there was no accompanying elevations in serum bilirubin and no patient developed clinically apparent liver injury with jaundice. The product label for sotorasib recommends monitoring for routine liver tests before, at 3 week intervals during the first 3 months of therapy, and monthly thereafter as clinically indicated. Strikingly, the more severe elevations of serum aminotransferase levels during therapy with sotorasib occurred among patients who had recently received checkpoint inhibitor therapy (usually anti-PD-L1) in the 1 to 3 months before starting sotorasib. Furthermore, the elevations tended to respond quickly to corticosteroid therapy and sometimes did not recur when sotorasib was restarted several months later. These findings suggest that the aminotransferase elevations during sotorasib therapy are due to a delayed immune-mediated hepatotoxicity triggered by the previous checkpoint inhibitor therapy. Likelihood score: D (possible but infrequent cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sotorasib during breastfeeding. Because sotorasib is 89% bound to plasma proteins, the amount in milk is likely to be low. However, because of its potential toxicity in the breastfed infant, the manufacturer recommends that breastfeeding be discontinued during sotorasib therapy and for 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sotorasib is 89% protein bound in plasma. |
| References | |
| Additional Infomation |
Sotorasib is a pyridopyrimidine that is pyrido[2,3-d]pyrimidin-2(1H)-one substituted by 4-methyl-2-(propan-2-yl)pyridin-3-yl, (2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl, fluoro and 2-fluoro-6-hydroxyphenyl groups at positions 1, 4, 6 and 7, respectively. It is approved for the treatment of patients with non-small cell lung cancer having KRAS(G12C) mutations. It has a role as an antineoplastic agent. It is a member of acrylamides, a N-acylpiperazine, a pyridopyrimidine, a member of monofluorobenzenes, a member of methylpyridines, a tertiary carboxamide, a tertiary amino compound and a member of phenols.
Sotorasib, also known as AMG-510, is an acrylamide-derived KRAS inhibitor developed by Amgen. It is indicated in the treatment of adult patients with KRAS G12C mutant non-small cell lung cancer. This mutation makes up >50% of all KRAS mutations. Mutant KRAS discovered in 1982 but was not considered a druggable target until the mid-2010s. It is the first experimental KRAS inhibitor. The drug [MRTX849] is also currently being developed and has the same target. Sotorasib was granted FDA approval on May 28, 2021, followed by the European Commission's approval on January 10, 2022. Sotorasib is a small molecule inhibitor of the KRAS G12C mutant protein which is found in up to 13% of refractory cases of non-small cell lung cancer. Serum aminotransferase elevations are common during therapy with sotorasib, and a proportion of patients develop clinically apparent liver injury that can be severe. Sotorasib is an orally available inhibitor of the specific KRAS mutation, p.G12C, with potential antineoplastic activity. Upon oral administration, sotorasib selectively targets, binds to and inhibits the activity of the KRAS p.G12C mutant. This may inhibit growth in KRAS p.G12C-expressing tumor cells. The KRAS p.G12C mutation is seen in some tumor cell types and plays a key role in tumor cell proliferation. Drug Indication Sotorasib is indicated in the treatment of KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults who have received at least one prior systemic therapy. Lumykras as monotherapy is indicated for the treatment of adults with advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutation and who have progressed after at least one prior line of systemic therapy. Mechanism of Action Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway. GTP is hydrolyzed to GDP, and KRAS is inactivated. KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. Sotorasib binds to the cysteine residue in KRAS G12C mutations, holding the protein in its inactive form. The cysteine residue that sotorasib targets is not present in the wild type KRAS, which prevents off-target effects. This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors. Pharmacodynamics Sotorasib is indicated in the treatment of adults with KRAS G12C mutant non small cell lung cancer. It has a moderate duration of action as it is given daily. Patients should be counselled regarding the risks of hepatotoxicity, interstitial lung disease and pneumonitis; and to avoid breastfeeding during treatment and up to 1 week after the last dose. |
| Molecular Formula |
C30H30F2N6O3
|
|---|---|
| Molecular Weight |
560.5944
|
| Exact Mass |
560.23
|
| Elemental Analysis |
C, 64.28; H, 5.39; F, 6.78; N, 14.99; O, 8.56
|
| CAS # |
2296729-00-3
|
| Related CAS # |
Sotorasib racemate; 2252403-56-6; Sotorasib isomer; Sotorasib-d7; 2296729-66-1; 2387559-45-5
|
| PubChem CID |
137278711
|
| Appearance |
White to yellow solid powder
|
| LogP |
4
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
41
|
| Complexity |
1030
|
| Defined Atom Stereocenter Count |
1
|
| SMILES |
C[C@H]1CN(CCN1C2=NC(=O)N(C3=NC(=C(C=C32)F)C4=C(C=CC=C4F)O)C5=C(C=CN=C5C(C)C)C)C(=O)C=C
|
| InChi Key |
NXQKSXLFSAEQCZ-SFHVURJKSA-N
|
| InChi Code |
InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1
|
| Chemical Name |
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one
|
| Synonyms |
AMG-510; sotorasib; AMG 510; AMG510; trade names: Lumakras; Lumykras;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: 50~100 mg/mL (89.2~178.4 mM)
Ethanol: ~13 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.71 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.71 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (3.71 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 5%DMSO+ 40%PEG300+ 5%Tween 80+ 50%ddH2O: 5.0mg/ml (8.92mM) Solubility in Formulation 5: 10 mg/mL (17.84 mM) in 20% HP-β-CD in Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7838 mL | 8.9192 mL | 17.8383 mL | |
| 5 mM | 0.3568 mL | 1.7838 mL | 3.5677 mL | |
| 10 mM | 0.1784 mL | 0.8919 mL | 1.7838 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04933695 | Active Recruiting |
Drug: Sotorasib | Non-small Cell Lung Cancer | Amgen | January 28, 2022 | Phase 2 |
| NCT05054725 | Active Recruiting |
Drug: RMC-4630 Drug: Sotorasib |
Non-Small Cell Lung Cancer | Revolution Medicines, Inc. | December 30, 2021 | Phase 2 |
| NCT05198934 | Active Recruiting |
Drug: Sotorasib Drug: Panitumumab Drug: Regorafenib |
Colorectal Cancer (CRC) |
Amgen | April 19, 2022 | Phase 3 |
| NCT05993455 | Active Recruiting |
Drug: Oral sotorasib + IV Panitumumab |
Efficacy | Korea University Anam Hospital |
July 3, 2023 | Phase 2 |
| NCT04303780 | Active Recruiting |
Drug: AMG 510 Drug: Docetaxel |
KRAS p, G12c Mutated / Advanced Metastatic NSCLC |
Amgen | June 4, 2020 | Phase 3 |
|
|---|
AMG 510 inhibits ERK phosphorylation and growth of KRASG12C-mutant tumours in vivo.Nature. 2019 Nov;575(7781):217-223. |
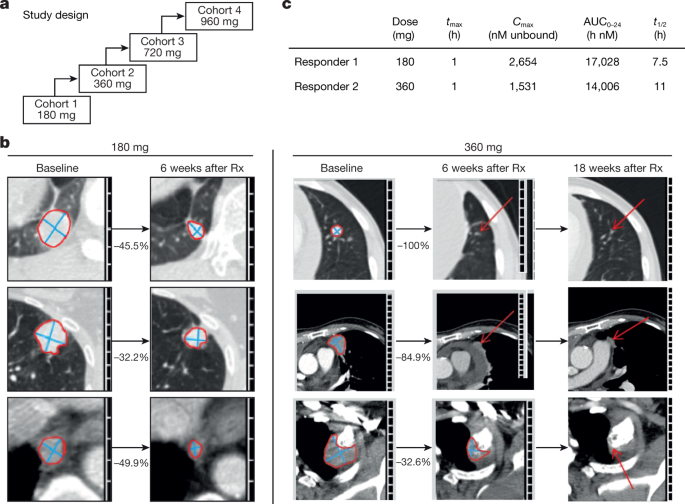 Clinical activity of AMG 510 in patients with lung cancer in first-in-human dose-escalation study.Nature. 2019 Nov;575(7781):217-223. Clinical activity of AMG 510 in patients with lung cancer in first-in-human dose-escalation study.Nature. 2019 Nov;575(7781):217-223. |