
| Size | Price | Stock | Qty |
|---|---|---|---|
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Amuvatinib (MP-470; HPK-56) is a novel, potent, orally bioavailable and multi-targeted inhibitor of c-Kit, PDGFRα and Flt3 with potential antineoplastic activity. It is a compound called carbothioamide that may have anti-tumor properties. By attaching itself to mutant variants of the stem cell factor receptor (c-Kit; SCFR), MP470 blocks the tyrosine kinase of this receptor that is clinically significant and may be linked to treatment resistance.
| Targets |
PDGFRαV561D (IC50 = 40 nM); PDGFRαD842V (IC50 = 81 nM); c-KitD816H (IC50 = 10 nM); c-KitV560G (IC50 = 34 nM); c-KitV654A (IC50 = 127 nM); c-KitD816V (IC50 = 950 nM)
|
|---|---|
| ln Vitro |
Amuvatinib (MP470) has IC50s of 950 nM, 10 nM, 34 nM, 127 nM, 81 nM, and 40 nM, respectively, for inhibiting c-Kit (D816V), c-Kit (D816H), c-Kit (V560G), c-Kit (V654A), and PDGFRα (D842V)[4].
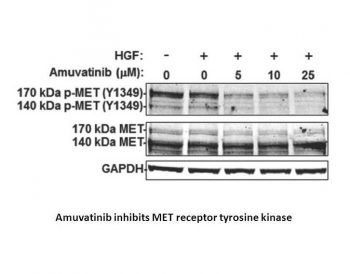
Amuvatinib (MP470), a novel inhibitor of receptor tyrosine kinase (RTK), Amuvatinib (MP470), has demonstrated growth inhibitory activity against multiple cancer cell lines. The LNCaP and PC-3 cells respond well to Amuvatinib (0.1–10 μM, incubated for 4 days), with IC50s of approximately 4 μM and 8 μM, respectively. When combined with different doses of Amuvatinib, Erlotinib (10 μM) causes the IC50 of Amuvatinib on LNCaP cells to drop to 2 μM[5]. Erlotinib or Imatinib Mesylate (IM) do not significantly lower Akt activity, but 10 μM Amuvatinib (treated for 30 hours) does. Akt activity is measured by phosphorylation on Ser473. Furthermore, in LNCaP cells with unaltered total Akt protein levels, amuvatinib plus erlotinib totally eliminated Akt phosphorylation[5]. |
| ln Vivo |
Doses of DMSO (control), Erlotinib 80 mg/kg, Amuvatinib (MP470) 50 mg/kg, or Erlotinib 80 mg/kg plus Amuvatinib 50 mg/kg daily are administered intraperitoneally to 12 mice in each of four LNCaP xenograft arms. The mice are then observed for an additional 11 days. Tumor growth inhibition (TGI) is only slightly inhibited by amuvatinib or erlotinib alone, but TGI is significantly affected (45–65%) by amuvatinib plus erlotinib therapy. However, only five or one mouse remained alive in the combination arm at the end of treatment or the study, respectively, because of the high doses of amuvatinib used. For the combination treatment, amuvatinib is therefore given at a lower dose of 10 mg/kg or 20 mg/kg. TGI in the group receiving 80 mg/kg of erlotinib plus 10 mg/kg of amuvatinib does not differ noticeably from that of the control group. In contrast to the control group, mice given 20 mg/kg Amuvatinib+80 mg/kg Erlotinib exhibited a significant TGI (p=0.01)[5].
|
| Enzyme Assay |
Various concentrations of MP-470 and radiolabeled γ-32P-ATP are incubated with enzymes to test their inhibitory activity against c-Kit and PDGFRα. The reaction mixtures are electrophoresed on an acrylamide gel after 30 minutes, and the amount of radioactivity that has been incorporated into the enzyme is used to measure autophosphorylation.
|
| Cell Assay |
On day zero, cells are plated in 96-well Falcon microtitier plates at a density of 2 × 103 to 1 × 104 cells per well in 100 μL medium. First, quadruplicates of ten μL of MP-470 serial dilutions are added to the plates. The cells are fixed using a 10% trichloroacetic acid solution after four days of incubation. They are then marked with 0.04% Sulforhodamine B (SRB) in 1% acetic acid. To dissolve the excess dye, 100 μL of a 50 mM Tris solution is added to each well after several washes. On a plate reader, the absorbance of every well is measured at 570 nm.
|
| Animal Protocol |
Forty eight 6-7 week-old SCID male mice with LNCaP xenograft model[2]
10 mg/kg and 20 mg/kg, 50 mg/kg Administered i.p. daily from days 1 to 24 |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Orally available |
| References |
|
| Additional Infomation |
N-(1,3-benzodioxol-5-ylmethyl)-4-(4-benzofuro[3,2-d]pyrimidinyl)-1-piperazinecarbothioamide is a N-arylpiperazine.
Amuvatinib has been used in trials studying the treatment of Solid Tumors and Small Cell Lung Carcinoma. Amuvatinib is an oral, selective multi-targeted tyrosine kinase inhibitor that suppresses c-MET, c-RET and the mutant forms of c-KIT, PDGFR and FLT3. Amuvatinib also suppresses Rad51 protein, a critical component of double-stranded DNA repair in cancer cells. Amuvatinib is an orally bioavailable synthetic carbothioamide with potential antineoplastic activity. Multitargeted receptor tyrosine kinase inhibitor MP470 binds to mutant forms of the stem cell factor receptor (c-Kit; SCFR), inhibiting clinically relevant mutants of this receptor tyrosine kinase that may be associated with resistance to therapy. In addition, MP470 inhibits activities of other receptor tyrosine kinases, such as c-Met, Ret oncoprotein, and mutant forms of Flt3 and PDGFR alpha, which are frequently dysregulated in variety of tumors. This agent also suppresses the induction of DNA repair protein Rad51, thereby potentiating the activities of DNA damage-inducing agents. Mutant forms of c-Kit are often associated with tumor chemoresistance. Drug Indication Amuvatinib is a selective multi-targeted tyrosine kinase inhibitor that suppresses c-MET, c-RET and the mutant forms of c-KIT, PDGFR and FLT3. Amuvatinib also suppresses Rad51 protein, a critical component of double-stranded DNA repair in cancer cells. |
| Molecular Formula |
C23H21N5O3S
|
|---|---|
| Molecular Weight |
447.51
|
| Exact Mass |
447.136
|
| Elemental Analysis |
C, 61.73; H, 4.73; N, 15.65; O, 10.73; S, 7.17
|
| CAS # |
850879-09-3
|
| Related CAS # |
Amuvatinib hydrochloride;1055986-67-8
|
| PubChem CID |
11282283
|
| Appearance |
White to off-white solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Boiling Point |
649.5±65.0 °C at 760 mmHg
|
| Flash Point |
346.6±34.3 °C
|
| Vapour Pressure |
0.0±1.9 mmHg at 25°C
|
| Index of Refraction |
1.739
|
| LogP |
2.79
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
3
|
| Heavy Atom Count |
32
|
| Complexity |
678
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S=C(N1CCN(C2C3OC4C(C=3N=CN=2)=CC=CC=4)CC1)NCC1C=C2C(OCO2)=CC=1
|
| InChi Key |
FOFDIMHVKGYHRU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H21N5O3S/c32-23(24-12-15-5-6-18-19(11-15)30-14-29-18)28-9-7-27(8-10-28)22-21-20(25-13-26-22)16-3-1-2-4-17(16)31-21/h1-6,11,13H,7-10,12,14H2,(H,24,32)
|
| Chemical Name |
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
|
| Synonyms |
Amuvatinib; MP470; HPK-56; MP-470; HPK 56; HPK56; MP 470
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.59 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 30% PEG400+0.5% Tween80+5% propylene glycol: 30mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2346 mL | 11.1729 mL | 22.3459 mL | |
| 5 mM | 0.4469 mL | 2.2346 mL | 4.4692 mL | |
| 10 mM | 0.2235 mL | 1.1173 mL | 2.2346 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01357395 | Completed | Drug: Amuvatinib | Small Cell Lung Carcinoma | Astex Pharmaceuticals, Inc. | May 2011 | Phase 2 |
| NCT00894894 | Completed | Drug: MP-470 Drug: amuvatinib (MP-470) |
Solid Tumors | Astex Pharmaceuticals, Inc. | May 2007 | Phase 1 |
| NCT00881166 | Completed | Drug: MP-470 + erlotinib Drug: MP-470 + docetaxel |
Malignant Disease | Astex Pharmaceuticals, Inc. | November 2007 | Phase 1 |
| NCT00504205 | Terminated | Other: pharmacological study Other: laboratory biomarker analysis |
Lymphoma Unspecified Adult Solid Tumor, Protocol Specific |
Astex Pharmaceuticals, Inc. | May 2007 | Not Applicable |
|
 |