
| Size | Price | Stock | Qty |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
Purity: ≥98%
AZD1390 (AZD-1390) is a novel, potent, selective, first-in-class orally bioavailable and CNS penetrant inhibitor of Ataxia-telangiectasia mutated (ATM) kinase with potential anticancer activity. In cell assays, it inhibits ATM with an IC50 of 0.78 nM. It exhibits excellent selectivity across a wide range of kinases and is >10,000 fold more selective than the closely related PIKK family of enzymes. Treating intracranial malignancies is appropriate for AZD1390 because it can cross the blood-brain barrier (BBB). Both lung cancer and glioma cell lines are radiosensitized by AZD1390; p53 mutant glioma cells are typically more radiosensitized than wild type. As a radiosensitizer for tumors of the central nervous system, AZD1390 is currently in the early stages of clinical research.
| Targets |
ATM ( IC50 = 0.78 nM )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
AZD1390 belongs to the same exquisitely potent series of ATM inhibitor as the clinical development compound AZD0156 (Fig. 1). However, AZD1390 was discovered following a series of in vitro assays designed to screen for (i) ATM autophosphorylation activity; (ii) selectivity against closely related PIKK family kinases ATR, DNA-PK, and mTOR activity and (iii) broader kinase panels; and (iv) lack of substrate activity in novel dual-transfected human MDR1 and BCRP efflux transporters assays. AZD1390 was screened against ATM [modulation of purified ATM-dependent phosphorylation of glutathione S-transferase (GST)–p53 Ser15] with activity defined as ≥50% [median inhibitory concentration (IC50)] of 0.00009 μM (0.00004 μM corrected for tight binding). IC50 activity against closely related and purified PIKK family enzymes was never more potent than 1 μM. In broader purified kinase screening panels, AZD1390 was tested at two concentrations, 1 and 0.1 μM, against the Thermo Fisher Scientific kinase panel. At the very high concentration of 1 μM, AZD1390 showed ≥50% inhibition against 3 targets (CSF1R, NUAK1, and SGK), with no activity against the remaining 118 targets tested. At 0.1 μM, no activity was found (<50% inhibition) against 354 kinases. We also tested activity and selectivity of AZD1390 against a panel of kinases run by Eurofins Panlabs. AZD1390 showed activity (>50% inhibition at 1 μM) against 1 kinase, FMS, and showed no activity (<50% inhibition at 1 μM) against 124 other kinases from the panel (Table 1). [2]
Brain and plasma binding of AZD1390[2] Rat brain binding (fubrain) was determined using the rat brain slice binding method, as detailed by Fridén et al.. Plasma binding (rat, mouse, dog, monkey, and human) was determined by equilibrium dialysis using a rapid equilibrium device. The compound in plasma at 1 or 0.1 μM was dialyzed with buffer at pH 7.4 and 37°C for 16 hours. After incubation, aliquots of both plasma and buffer were added to equal volumes of blank buffer and plasma, respectively, before precipitation with acetonitrile prior to centrifugation and analysis of the supernatants by UPLC-MS/MS. Fuplasma was determined by dividing the concentration in the buffer chamber by the concentration in the plasma chamber. |
|
| Cell Assay |
In an RPMI format, 3000 cells per well are seeded using 10% fetal bovine serum in a 384-well format.Plates are Echo-dosed with a semi-log dose dilution of each compound after a 24-hour period, starting at a top concentration of 1250 nM. After compound dosing, plates are exposed to 0, 2.5, or 4 Gy of radiation for one hour. After the plates are fixed at 1, 6, 24, and 48 hours after the radiation, they are incubated for 30 minutes at room temperature and then three times with phosphate-buffered saline solution (PBSA). This is done by directly adding a 1:1 volume of 8% PFA to the medium, resulting in a final concentration of 4% PFA.
|
|
| Animal Protocol |
|
|
| ADME/Pharmacokinetics |
BBB penetration[2]
The endothelial cells of the BBB contain efflux transporters MDR1 (Pgp) and BCRP, which serve to actively exclude the compound from the brain (32). In vitro efflux assays were set up using Madin-Darby canine kidney (MDCK) cells dual-transfected with human MDR1 and BCRP efflux transporters to identify compounds without substrate activity. In vitro MDCK_MDR1_BCRP studies at both 1 and 0.1 μM suggest that AD1390 is not a substrate for the human Pgp and/or BCRP efflux transporters (efflux ratio, <2); however, it does have a higher efflux rate in rodent species, as lower Kp,uu values were observed in rat and mouse (0.17 and 0.04, respectively). This reflects that, in rodents, AZD1390 is seen to be an efflux substrate with increased brain exposure (Kp,uu, 0.85 and 0.77) on administration of the chemical efflux transporter knockout elacridar (Fig. 1C) and an efflux ratio of 3.2 in the rat transporter-transfected in vitro LLC-PK1-rMdr1a assay at 1 μM. In contrast, AZD0156 at 0.1 μM has an efflux ratio of 23, indicating that it is a human efflux transporter substrate (Fig. 1, B and C). This BBB permeability difference is also reflected in vivo with AZD1390 rat and mouse brain Kp,uu values six- and sevenfold higher, respectively, than AZD0156. Cynomolgus macaque positron emission tomography (PET) images for the two compounds (Fig. 1D) show that only AZD1390 gives significant brain penetration with a Cmax (%ID) of 0.68 ± 0.078 (n = 5) [compare AZD0156 Cmax %ID 0.15 ± 0.036 (n = 3, P < 0.01)]. Two-tissue compartment (2-TC) modeling of AZD1390 PET data yielded a VT (equivalent to Kp) of 5.8 ± 1.2 (n = 5) and a calculated Kp,uu of 0.33 ± 0.068 (n = 5). It was not possible to accurately determine Kp for AZD0156 in cynomolgus macaques. Here, the 2-TC model showed poor identifiability with very high SEs in VT. We observed lower Kp,uu values in rat and mouse for AZD1390 (0.17 and 0.04, respectively). This reflects that, in rodents, AZD1390 appears to be an efflux substrate with increased brain exposure (Kp,uu, 0.85 and 0.77) on administration of the chemical efflux transporter knockout elacridar (Fig. 3B) and an efflux ratio of 3.2 in the rat transporter-transfected in vitro LLC-PK1-rMdr1a assay at 1 μM. Despite the lower rodent Kp,uu values in mouse at 2 to 20 mg/kg, free brain exposure is achieved, with pATM inhibition and efficacy observed.[2] PD and PK of AZD1390 in vivo[2] Researchers performed an extensive assessment of the relationship between PK and PD of AZD1390 in plasma, brain, and tumor samples from our orthotopic brain tumor model, NCI-H2228, implanted in the brain. The data show that the combination of pharmacologically active doses of AZD1390 from the in vitro and cell potency assays inhibited the IR-induced PD biomarkers pATM (Ser1981) and phospho-Rad50 (pRad50) (Ser635) in vivo in a dose- and time-dependent manner (Fig. 3, A and B). The antibody used to detect the latter is being used in clinical trials, and the data in Fig. 4B show the staining levels correlating with PK observations in Fig. 2A. The combination of AZD1390 with IR also significantly increased the apoptotic marker CC3 (cleaved caspase-3) compared to IR alone in NCI-H2228 lung cancer brain metastasis (LC-BM) model, suggesting that the combination is inducing tumor cell death (Fig. 3C). The data reveal a correlation between PK and PD modulation, with AZD1390 free brain levels peaking within 1 hour of dosing and dissipating over a 24-hour period, correlating with ATM inhibition activity (see fig. S4, A and D, for further details on PK and PD analyzed). |
|
| Toxicity/Toxicokinetics |
The IC50 against the cardiac ion channel hERG was also confirmed as minimal for both AZD0156 and AZD1390: >33.3 and 6.55 μM, respectively. (A similar IC50 for AZD1390 of 7.99 μM against hERG was generated using an alternative assay with improvements in compound handling and data processing).[2]
|
|
| References | ||
| Additional Infomation |
ATM Kinase Inhibitor AZD1390 is an orally bioavailable inhibitor of ataxia telangiectasia mutated (ATM) kinase, with potential antineoplastic activity. Upon oral administration, AZD1390 targets and binds to ATM, thereby inhibiting the kinase activity of ATM and ATM-mediated signaling. This prevents DNA damage checkpoint activation, disrupts DNA damage repair, induces tumor cell apoptosis, and leads to cell death in ATM-overexpressing tumor cells. AZD1390 hypersensitizes tumors to chemo/radiotherapy. In addition, AZD1390 is able to cross the blood-brain barrier (BBB). ATM, a serine/threonine protein kinase belonging to the phosphatidylinositol 3-kinase-related kinase (PIKK) family of protein kinases, is upregulated in a variety of cancer cell types. It is activated in response to DNA double-strand breaks (DSB) and plays a key role in DNA repair.
|
| Molecular Formula |
C27H32FN5O2
|
|
|---|---|---|
| Molecular Weight |
477.5737
|
|
| Exact Mass |
477.25
|
|
| Elemental Analysis |
C, 67.90; H, 6.75; F, 3.98; N, 14.66; O, 6.70
|
|
| CAS # |
2089288-03-7
|
|
| Related CAS # |
|
|
| PubChem CID |
126689157
|
|
| Appearance |
White to off-white solid powder
|
|
| LogP |
4.2
|
|
| Hydrogen Bond Donor Count |
0
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
7
|
|
| Heavy Atom Count |
35
|
|
| Complexity |
720
|
|
| Defined Atom Stereocenter Count |
0
|
|
| InChi Key |
VQSZIPCGAGVRRP-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C27H32FN5O2/c1-18(2)33-26-21-14-20(22(28)15-23(21)29-17-24(26)31(3)27(33)34)19-8-9-25(30-16-19)35-13-7-12-32-10-5-4-6-11-32/h8-9,14-18H,4-7,10-13H2,1-3H3
|
|
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0939 mL | 10.4697 mL | 20.9393 mL | |
| 5 mM | 0.4188 mL | 2.0939 mL | 4.1879 mL | |
| 10 mM | 0.2094 mL | 1.0470 mL | 2.0939 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04550104 | Recruiting | Drug: AZD1390 Drug: TBD Compound 1 |
Non Small Cell Lung Cancer | University of Leeds | March 17, 2021 | Phase 1 |
| NCT05116254 | Recruiting | Combination Product: AZD1390 + radiotherapy |
Soft Tissue Sarcoma Adult | The Netherlands Cancer Institute | July 18, 2022 | Phase 1 |
| NCT05678010 | Recruiting | Radiation: Stereotactic Body Radiotherapy Drug: AZD1390 |
Solid Tumor Solid Carcinoma |
Memorial Sloan Kettering Cancer Center |
May 17, 2023 | Phase 1 |
| NCT03423628 | Recruiting | Radiation: Radiation Therapy Drug: AZD1390 |
Brain Neoplasms, Malignant Leptomeningeal Disease (LMD) |
AstraZeneca | April 2, 2018 | Phase 1 |
| NCT05182905 | Recruiting | Drug: AZD1390 | Glioblastoma Glioma |
Nader Sanai | March 9, 2022 | Early Phase 1 |
The structure and brain-penetrating properties of AZD1390.Science Advances. 2018, 4(6): eaat1719. |
|---|
Target engagement and cellular mechanism of action of AZD1390.Science Advances. 2018, 4(6): eaat1719. |
In vivo activity of AZD1390 in lung-brain metastatic models.Science Advances. 2018, 4(6): eaat1719. |
Survival of a syngeneic mouse model of GBM treated with AZD1390.Science Advances. 2018, 4(6): eaat1719. |
|---|
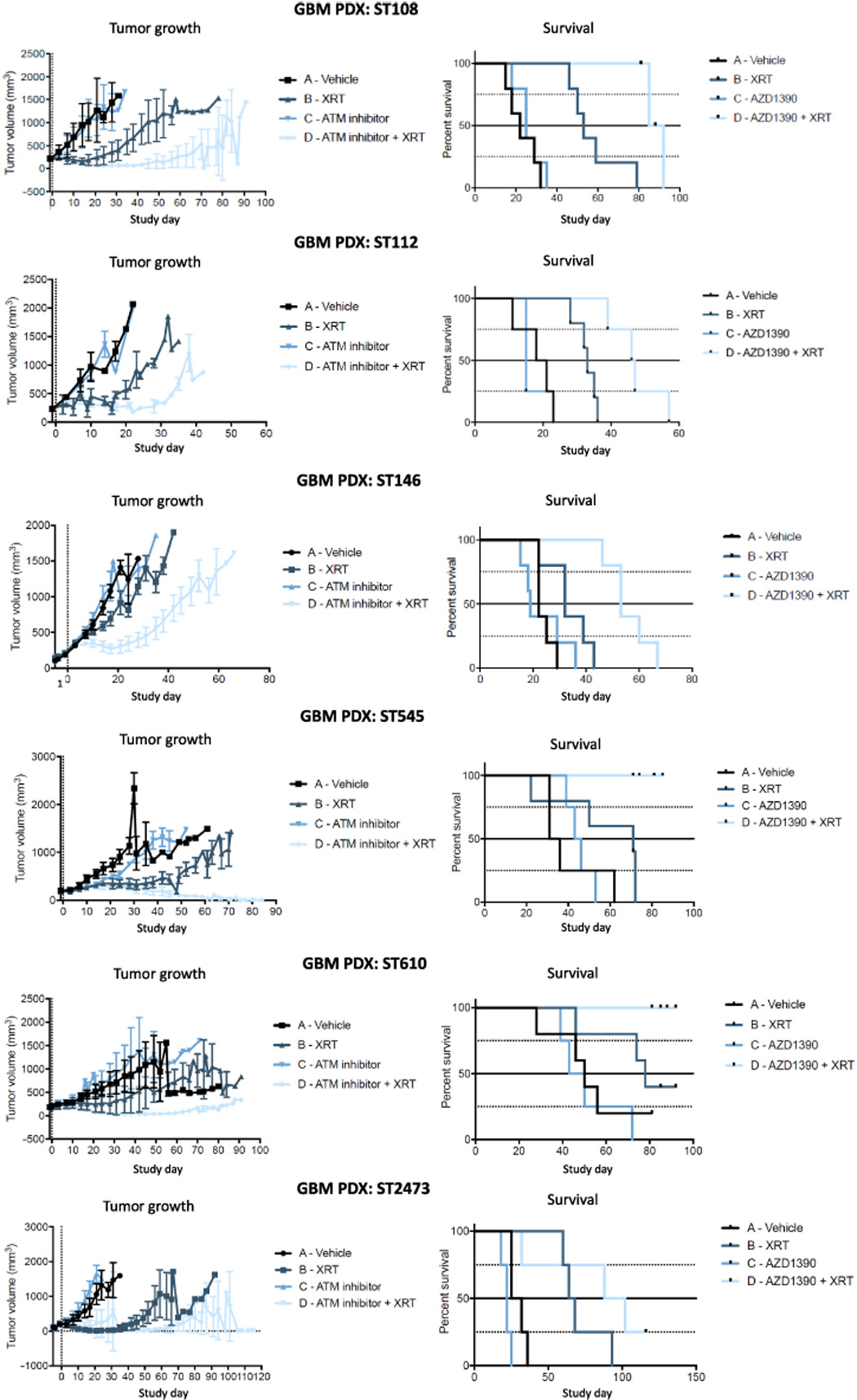 In vivo subcutaneous efficacy studies using PDX models.Science Advances. 2018, 4(6): eaat1719. |
harmacokinetics and pharmacodynamicsof AZD1390.Science Advances. 2018, 4(6): eaat1719. |