
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Bepotastine besilate (bepotastine besylate; TAU-284; TAU284; Bepreve; Talion), the besylate salt of Bepotastine, is a 2nd generation, non-sedating, and selective antagonist of histamine 1 (H1) receptor with a pIC50 of 5.7. Bepostatine besilate has been given approval to treat pruritus, urticaria, and allergic rhinitis. By blocking histamine H1 receptors, antagonistically opposing the vasoconstrictor and, to a lesser degree, the vasodilator effects of histamine, bepotastine besilate inhibits the action of histamine. Mast cell stabilisers work by stopping the regular flow of intracellular signals, which inhibits degranulation and the subsequent release of histamine. Talion is the brand name for bepostatine besilate.
| Targets |
Histamine H1 receptor ( pIC50 = 5.7 )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Cell Assay |
Cell Line: NHEKs
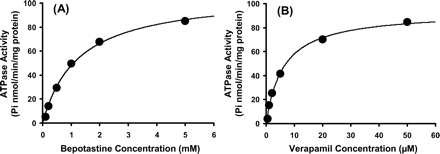
Concentration: 50 µM (preincubation) Incubation Time: 1 h Result: Suppressed the expression of NGF mRNA in NHEKs. |
|
| Animal Protocol |
Guinea pigs (6-week-old)
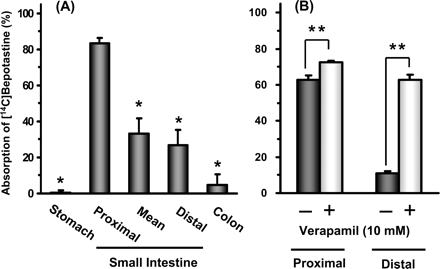
10 g/L (1.0% (w/v)) for 10 µL Eye drop; 3 times at intervals of 20 min (in one eye). |
|
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
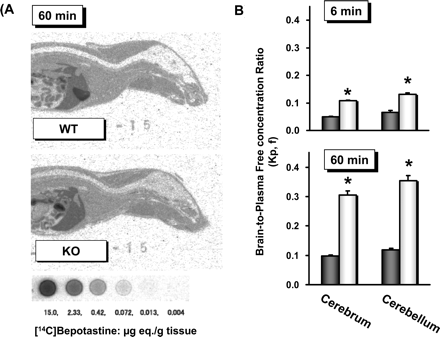
◉ Summary of Use during Lactation There are no reports of infants breastfed during maternal therapy with bepotastine. Because absorption from the eye is limited, bepotastine would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information on bepotastine was not found as of the revision date. In one telephone follow-up study, mothers reported irritability and colicky symptoms in 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention and none of the patients were using bepotastine. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Low ophthalmic doses of bepotastine are unlikely to have the same effect on serum prolactin. |
|
| References |
|
|
| Additional Infomation |
Bepotastine besylate is an organosulfonate salt obtained by combining equimolar amounts of bepotastine and benzenesulfonic acid. A topical, selective and non-sedating histamine (H1) receptor antagonist used for treatment of itching associated with allergic conjunctivitis. It has a role as a H1-receptor antagonist and an anti-allergic agent. It contains a bepotastine(1+).
See also: Bepotastine (annotation moved to). |
| Molecular Formula |
C27H31CLN2O6S
|
|
|---|---|---|
| Molecular Weight |
547.06
|
|
| Exact Mass |
546.159
|
|
| Elemental Analysis |
C, 59.28; H, 5.71; Cl, 6.48; N, 5.12; O, 17.55; S, 5.86
|
|
| CAS # |
190786-44-8
|
|
| Related CAS # |
(Rac)-Bepotastine besilate; 1415692-17-9; Bepotastine; 125602-71-3; Bepotastine tosylate; 1160415-45-1
|
|
| PubChem CID |
164521
|
|
| Appearance |
Off-white to light yellow solid powder
|
|
| Boiling Point |
546.8ºC at 760 mmHg
|
|
| Melting Point |
161-163°
|
|
| Flash Point |
284.5ºC
|
|
| Vapour Pressure |
8.63E-13mmHg at 25°C
|
|
| LogP |
6.122
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
9
|
|
| Heavy Atom Count |
37
|
|
| Complexity |
632
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])[C@@]([H])(C1=C([H])C([H])=C([H])C([H])=N1)OC1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])C(=O)O[H])C([H])([H])C1([H])[H].S(C1C([H])=C([H])C([H])=C([H])C=1[H])(=O)(=O)O[H]
|
|
| InChi Key |
UDGHXQPQKQPSBB-BOXHHOBZSA-N
|
|
| InChi Code |
InChI=1S/C21H25ClN2O3.C6H6O3S/c22-17-8-6-16(7-9-17)21(19-4-1-2-12-23-19)27-18-10-14-24(15-11-18)13-3-5-20(25)26;7-10(8,9)6-4-2-1-3-5-6/h1-2,4,6-9,12,18,21H,3,5,10-11,13-15H2,(H,25,26);1-5H,(H,7,8,9)/t21-;/m0./s1
|
|
| Chemical Name |
benzenesulfonic acid;4-[4-[(S)-(4-chlorophenyl)-pyridin-2-ylmethoxy]piperidin-1-yl]butanoic acid
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.57 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.57 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.57 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8280 mL | 9.1398 mL | 18.2795 mL | |
| 5 mM | 0.3656 mL | 1.8280 mL | 3.6559 mL | |
| 10 mM | 0.1828 mL | 0.9140 mL | 1.8280 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03932435 | Completed | Drug: Fisrt period TWLO_C Other: Washout period |
Healthy | Dong-A ST Co., Ltd. | April 23, 2019 | Not Applicable |
| NCT01840605 | Completed | Drug: Bepotastine besilate Drug: ketotifen fumarate |
Dermatitis Atopic |
Mitsubishi Tanabe Pharma Corporation | March 2013 | Phase 3 |
| NCT01753739 | Completed | Drug: Bepotastine besilate Drug: Placebo |
Seasonal Allergic Rhinitis | Bausch & Lomb Incorporated | January 2013 | Phase 2 |
| NCT01861522 | Completed | Drug: Bepotastine besilate Drug: Placebo |
Perennial Allergic Rhinitis | Mitsubishi Tanabe Pharma Corporation |
April 2013 | Phase 3 |
| NCT01900054 | Completed | Drug: Bepotastine besilate | Perennial Allergic Rhinitis | Mitsubishi Tanabe Pharma Corporation |
June 2013 | Phase 3 |
 |
|---|
 |
 |