
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
BI2536 (BI-2536; BI 2536) is a novel PLK1/BRD4 dual inhibitor with potential antitumor activity. In a cell-free assay, it inhibits PLK1/BRD4 with IC50s of 0.83 and 25 nM, respectively. The serine/threonine protein kinase Plk1 is an essential regulator of several processes essential to cell division and mitosis.
| Targets |
PLK1 (IC50 = 0.83 nM); Plk2/Snk (IC50 = 3.5 nM); Plk3/Fnk (IC50 = 9 nM); BRD4 (IC50 = 25 nM)
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
Recombinant human Plk1 (residues 1–603) is purified by affinity chromatography using glutathione-agarose after being expressed as an N-terminal, GST-tagged fusion protein using a baculoviral expression system. When conducting Plk1 enzyme activity assays, serially diluted BI 2536 is present along with 20 ng of recombinant kinase and 10 μg of casein from cow's milk used as the substrate. A final volume of 60 μL is used for kinase reactions, which are carried out for 45 minutes at 30 °C (15 mM MgCl2, 25 mM MOPS [pH 7.0], 1 mM DTT, 1% DMSO, 7.5 mM ATP, 0.3 μCi γ-33P-ATP). A final 125 μL of ice-cold 5% TCA is added to stop the reaction. Following the transfer of precipitates to Multi-Screen mixed ester cellulose filter plates, the plates undergo a 1% TCA wash and radiometric quantification. The IC50 value is computed using the dose-response curve.
|
|
| Cell Assay |
In assays for cell proliferation, different concentrations of BI 2536 (10 nM-1 μM) are added and incubated for 72 hours. The growth of the cells is measured by measuring the Alamar Blue dye conversion in a fluorescence spectrophotometer. From the dose-response curve fit, effective concentrations (EC50) at which cellular growth is 50% inhibited are extrapolated[3].
|
|
| Animal Protocol |
Mice: Subcutaneous injection of 2×106, 1×106, or 1×107 cells into each mouse's flank results in the grafting of HCT 116 colon-carcinoma, NCI-H460, or A549 lung-carcinoma cells into female BomTac:NMRI-Foxn1nu mice. Animals are paired into groups of ten mice each for treatment and control once tumors have grown to a volume of about 50 mm3. Treatment is not started in regression experiments until the mean tumor volume reaches 500 mm3. The recommended dosage and schedule for intravenous injection of BI 2536 are administered into the tail vein. Ten milliliters for every kilogram of body weight is the dosage. Three times a week, calipers are used to determine tumor volumes. The following formula is used to convert the results to tumor volume (mm3): length×width2×π/6. On the same days, the mice's weight is ascertained as a measure of tolerability. In a one-sided (decreasing) exact Wilcoxon test, the treatment group and the vehicle control group are compared for statistical analysis.
|
|
| References |
|
|
| Additional Infomation |
BI 2536 is under investigation in clinical trial NCT00376623 (Efficacy and Safety of BI 2536 in Advanced or Metastatic Non Small Cell Lung Cancer).
Plk1 Inhibitor BI 2536 is a small molecule compound with potential antineoplastic activities. BI 2536 binds to and inhibits Polo-like kinase 1 (Plk1), resulting in mitotic arrest, disruption of cytokinesis, and apoptosis in susceptible tumor cell populations. Plk1, a serine/threonine-protein kinase, is a key regulator of multiple processes fundamental to mitosis and cell division. |
| Molecular Formula |
C28H39N7O3
|
|
|---|---|---|
| Molecular Weight |
521.66
|
|
| Exact Mass |
521.311
|
|
| Elemental Analysis |
C, 64.47; H, 7.54; N, 18.80; O, 9.20
|
|
| CAS # |
755038-02-9
|
|
| Related CAS # |
|
|
| PubChem CID |
11364421
|
|
| Appearance |
Off-white to yellow solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Index of Refraction |
1.634
|
|
| LogP |
1.81
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
7
|
|
| Heavy Atom Count |
38
|
|
| Complexity |
816
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
O=C1[C@@]([H])(C([H])([H])C([H])([H])[H])N(C2C(=C([H])N=C(N([H])C3C([H])=C([H])C(=C([H])C=3OC([H])([H])[H])C(N([H])C3([H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C3([H])[H])=O)N=2)N1C([H])([H])[H])C1([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H]
|
|
| InChi Key |
XQVVPGYIWAGRNI-JOCHJYFZSA-N
|
|
| InChi Code |
InChI=1S/C28H39N7O3/c1-5-22-27(37)34(3)23-17-29-28(32-25(23)35(22)20-8-6-7-9-20)31-21-11-10-18(16-24(21)38-4)26(36)30-19-12-14-33(2)15-13-19/h10-11,16-17,19-20,22H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t22-/m1/s1
|
|
| Chemical Name |
4-[[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-7H-pteridin-2-yl]amino]-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3.25 mg/mL (6.23 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3.25 mg/mL (6.23 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (3.99 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 30%PEG 400 +0.5% Tween 80 +5% Propylene glycol : 15mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9170 mL | 9.5848 mL | 19.1696 mL | |
| 5 mM | 0.3834 mL | 1.9170 mL | 3.8339 mL | |
| 10 mM | 0.1917 mL | 0.9585 mL | 1.9170 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00412880 | Completed | Drug: BI 2536 | Carcinoma, Small Cell | Boehringer Ingelheim | February 14, 2007 | Phase 2 |
| NCT00243087 | Completed | Drug: BI 2536 | Lymphoma | Boehringer Ingelheim | July 2005 | Phase 1 |
| NCT00706498 | Completed | Drug: BI 2536 | Prostatic Neoplasms | Boehringer Ingelheim | September 2006 | Phase 2 |
|
|---|
Crystal structure of BI-2536 bound to BRD4(1) (PDB ID = 4OGI).ACS Med Chem Lett.2015 May 18;6(7):764-9. |
\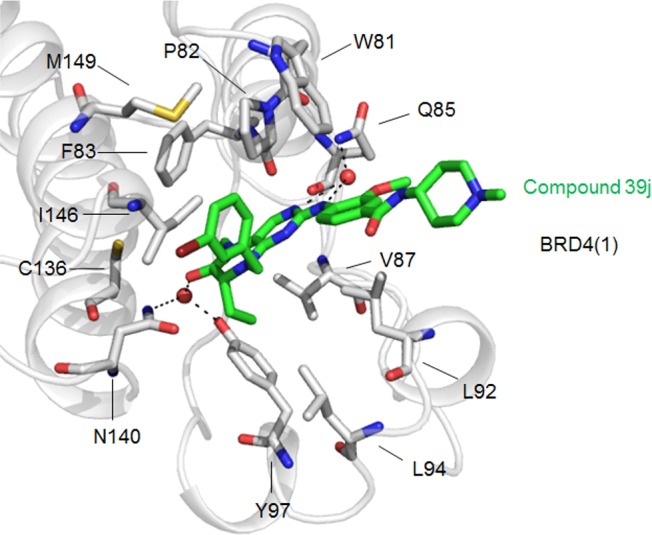 High scoring, GOLD-predicted docking mode of compound39jin BRD4(1).ACS Med Chem Lett.2015 May 18;6(7):764-9. |