
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
BX795 is a novel, potent and selective/specific PDK-1 (3-phosphoinositide-dependent kinase-1) inhibitor with potential antitumor activity. In cell-free assays, it exhibits 140- and 1600-fold more selectivity for PDK1 over PKA and PKC, respectively, and inhibits PDK-1 with an IC50 of 6 nM. High antitumor efficacy and potent antiproliferative activity were demonstrated by BX795 in vivo. In a direct kinase assay format, it works by attaching to the ATP-binding pocket of PDK1 and thereby potently inhibiting the enzyme's activity. In macrophages stimulated with poly(I:C) or lipopolysaccharide (LPS), BX795 inhibits the phosphorylation, nuclear translocation, and transcriptional activity of interferon regulatory factor 3 as well as the production of interferon-β.
| Targets |
PDPK1 (IC50 = 6 nM); c-Kit (IC50 = 320 nM); CDK2/CyclinE (IC50 = 430 nM); Chk1 (IC50 = 510 nM)
|
|---|---|
| ln Vitro |
BX-795 efficiently inhibits PDK1 activity in PC-3 cells as evidenced by their capacity to prevent S6K1, Akt, PKCδ, and GSK3β phosphorylation. BX-795 has an effective inhibitory effect on tumor cell growth on plastic, with IC50 values for MDA-468, HCT-116, and MiaPaca cells of 1.6, 1.4, and 1.9 M, respectively. BX-795 exhibits greater growth inhibition in soft agar, with IC50 values for MDA-468 and PC-3 cells of 0.72 and 0.25 μM, respectively.[1]
BX-795 an inhibitor of TBK1/IKKɛ, also prevents the activation of IRF3 and the production of IFN-β by TBK1 and IKKɛ.[2] BX795 inhibits the production of ATP, thromboxane, and 2-MeSADP-induced or collagen-induced aggregation in platelet physiological responses.[3] |
| ln Vivo |
BX795, a TBK-1 and PDK-1 inhibitor, also inhibits HSV protein translation.
|
| Enzyme Assay |
PDK1 is assayed in a direct kinase assay and a coupled assay format measuring PDK1- and PtdIns-3,4-P2-mediated activation of AKT2. For the coupled assay, the final assay mixture (60 μL) contained: 15 mM MOPS, pH 7.2, 1 mg/mL bovine serum albumin, 18 mM β-glycerol phosphate, 0.7 mM dithiothreitol, 3 mM EGTA, 10 mM MgOAc, 7.5 μM ATP, 0.2 μCi of [γ-33P]ATP, 7.5 μM biotinylated peptide substrate (biotin-ARRRDGGGAQPFRPRAATF), 0.5 μL of PtdIns-3,4-P2-containing phospholipid vesicles, 60 pg of purified recombinant human PDK1, and 172 ng of purified recombinant human AKT2. The biotin-labeled peptide is captured from 10 μl of the assay mixture on streptavidin-coated SPA beads after 2 hours of room temperature incubation, and product formation is determined by scintillation proximity in a Wallac MicroBeta counter. The amount of PDK1 and inactive AKT2 added, as well as the incubation period, all influence the final product. In order for the assay to sensitively identify both direct inhibitors of PDK1 or AKT1 as well as inhibitors of AKT2 activation, PDK1 is added at suboptimal levels. To measure PDK1 activity directly, the final assay mixture (60 μL) contained 50 mM Tris-HCl, pH 7.5, 0.1 mM EGTA, 0.1 mM EDTA, 0.1% β-mercaptoethanol, 1 mg/mL bovine serum albumin, 10 mM MgOAc, 10 μM ATP, 0.2 μCi of [γ-33P]ATP, 7.5 μM substrate peptide (H2N-ARRRGVTTKTFCGT), and 60 ng of purified recombinant human PDK1. We added 25 mM EDTA and spotted a portion of the reaction mixture on Whatman P81 phosphocellulose paper after the mixture had been at room temperature for 4 hours.
|
| Cell Assay |
Low density cells (1,500–3,000 cells/well, 0.1 mL/well, 96-well plates) are incubated overnight. Compound treatments are created by adding 10 μL
/well of the compound in 1% dimethyl sulfoxide and growth medium (final concentration of dimethyl sulfoxide, 0.1%), followed by a brief shaking. The viability of the treated cells is assessed after a 72-hour incubation period by adding 10 μL of the metabolic dye WST-1. The net signal is calculated by subtracting a no cell, or zero time cell, background from the WST-1 signal, which is read at 450 nm in a plate reader. Results are presented as the mean ± S.E. of two or more replicates.
|
| Animal Protocol |
NA;
|
| References | |
| Additional Infomation |
N-[3-[[5-iodo-4-[3-[[oxo(thiophen-2-yl)methyl]amino]propylamino]-2-pyrimidinyl]amino]phenyl]-1-pyrrolidinecarboxamide is a member of ureas.
|
| Molecular Formula |
C23H26IN7O2S
|
|---|---|
| Molecular Weight |
591.4677
|
| Exact Mass |
591.091
|
| Elemental Analysis |
C, 46.71; H, 4.43; I, 21.46; N, 16.58; O, 5.41; S, 5.42
|
| CAS # |
702675-74-9
|
| Related CAS # |
702675-74-9
|
| PubChem CID |
10077147
|
| Appearance |
White to light brown solid powder
|
| Density |
1.6±0.1 g/cm3
|
| Index of Refraction |
1.738
|
| LogP |
2.73
|
| Hydrogen Bond Donor Count |
4
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
9
|
| Heavy Atom Count |
34
|
| Complexity |
669
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
IC1=C([H])N=C(N=C1N([H])C([H])([H])C([H])([H])C([H])([H])N([H])C(C1=C([H])C([H])=C([H])S1)=O)N([H])C1C([H])=C([H])C([H])=C(C=1[H])N([H])C(N1C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O
|
| InChi Key |
VAVXGGRQQJZYBL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H26IN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30)
|
| Chemical Name |
N-[3-[[5-iodo-4-[3-(thiophene-2-carbonylamino)propylamino]pyrimidin-2-yl]amino]phenyl]pyrrolidine-1-carboxamide
|
| Synonyms |
BX 795; BX-795; BX795
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (~169.1 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.23 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.23 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% propylene glycol: 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6907 mL | 8.4535 mL | 16.9070 mL | |
| 5 mM | 0.3381 mL | 1.6907 mL | 3.3814 mL | |
| 10 mM | 0.1691 mL | 0.8454 mL | 1.6907 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
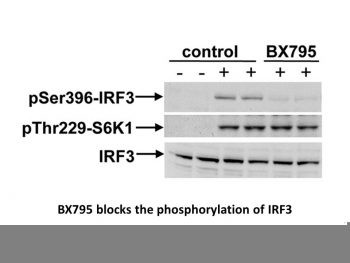 |
BX795 blocks the phosphorylation, nuclear translocation, and transcriptional activity of IRF3 and production of interferon β in response to TLR3 and TLR4 agonists.J Biol Chem.2009 May 22;284(21):14136-46. |
BX795 selectively blocks IRF3 but not NFκB signaling.J Biol Chem.2009 May 22;284(21):14136-46. |
The overexpression of TBK1 and IKKε leads to autophosphorylation and transphosphorylation of Ser-172.J Biol Chem.2009 May 22;284(21):14136-46. |
BX795 increases the phosphorylation of Ser-172 and the catalytic activity of TBK1 and IKKε in response to LPS and poly(I:C).J Biol Chem.2009 May 22;284(21):14136-46. |