| Size | Price | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| Targets |
β-1/2/3 adrenergic receptor
|
|---|---|
| ln Vitro |
At the β1-adrenoceptor, CGP 12177 potently antagonizes agonist responses at the primary high-affinity catecholamine conformation while also exerting agonist effects of its own through a secondary low-affinity conformation. A recent mutagenesis study identified transmembrane region (TM)4 of the β1-adrenoceptor as key for this low-affinity conformation. Others suggested that TM4 has a role in β1-adrenoceptor oligomerization. Here, assessment of the dissociation rate of a fluorescent analog of CGP 12177 [bordifluoropyrromethane-tetramethylrhodamine-(±)CGP 12177 (BODIPY-TMR-CGP)] at the human β1-adrenoceptor expressed in Chinese hamster ovary cells revealed negative cooperative interactions between 2 distinct β1-adrenoceptor conformations. The dissociation rate of 3 nM BODIPY-TMR-CGP was 0.09 ± 0.01 min(-1) in the absence of competitor ligands, and this was enhanced 2.2- and 2.1-fold in the presence of 1 µM CGP 12177 and 1 µM propranolol, respectively. These effects on the BODIPY-TMR-CGP dissociation rate were markedly enhanced in β1-adrenoceptor homodimers constrained by bimolecular fluorescence complementation (9.8- and 9.9-fold for 1 µM CGP 12177 and 1 µM propranolol, respectively) and abolished in β1-adrenoceptors containing TM4 mutations vital for the second conformation pharmacology. This study suggests that negative cooperativity across a β1-adrenoceptor homodimer may be responsible for generating the low-affinity pharmacology of the secondary β1-adrenoceptor conformation[1].
|
| ln Vivo |
This study investigates the effect of the aryloxypropanolamines 4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one (CGP 12177), bupranolol, and 3-(2-ethylphenoxy)-1[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate (SR 59230A) [commonly used as beta(3)- and/or atypical beta-adrenergic receptors (beta-AR) ligands] on the contractile function of rat intralobar pulmonary artery. Affinities of beta-AR ligands for alpha(1)-adrenergic receptors (alpha(1)-AR) were also evaluated using [(3)H]prazosin binding competition experiments performed in rat cortical membranes. In intralobar pulmonary artery, CGP 12177 did not modify the basal tone, but antagonized the contraction induced by the alpha(1)-AR agonist phenylephrine (PHE). In arteries precontracted with PHE, CGP 12177 elicited relaxation, whereas in those precontracted with prostaglandin F(2alpha) (PGF(2alpha)), it further enhanced contraction. CGP 12177 induced an increase in intracellular calcium concentration in pressurized arteries loaded with Fura PE-3 and precontracted with PGF(2alpha). In PGF(2alpha) precontracted arteries, phentolamine (an alpha-AR antagonist) and phenoxybenzamine (an irreversible alpha-AR antagonist) antagonized the contractile responses to PHE and CGP 12177. Both responses were also decreased by bupranolol and SR 59230A. Specific [(3)H]prazosin binding was displaced by CGP 12177, bupranolol, and SR 59230A with pK(i) values of 5.2, 5.7, and 6.6, respectively. In contrast, (+/-)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid sodium (BRL 37344) and disodium 5-[(2R)-2-([(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino)propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316243) (nonaryloxypropanolamines beta(3)-AR agonists) displayed very low affinity for [(3)H]prazosin binding sites (pK(i) values below 4). These data suggest that CGP 12177 exhibits partial agonist properties for alpha(1)-AR in rat pulmonary artery. They also show that bupranolol and SR 59230A exert an alpha(1)-AR antagonist effect. As a consequence, these aryloxypropanolamine compounds should be used with caution when investigating the role of beta(3)- and atypical beta-AR in the regulation of vascular tone [3].
|
| Enzyme Assay |
Background: Although S-(-)[C]CGP-12177 is a useful positron emission tomography (PET) ligand for beta-adrenoreceptors, the difficulty in radiolabelling the compound has prevented its extensive clinical application. Recently, we have developed a simple synthesis method for S-(-)[C]CGP-12177. In the present study, we attempted to prepare S-(-)[C]CGP-12177 with a high specific activity for intravenous injection which is feasible for the clinical evaluation of beta-adrenoreceptors.
Methods: The [C]methane produced during irradiation of a N2--H2 (95/5) mixture with an 18 MeV proton beam (20 microA, 30 min) was chlorinated using Cl2 to yield [C]carbon tetrachloride. S-(-)[C]CGP-12177 was synthesized by reacting the diamino precursor with [C]phosgene produced by oxidizing [C]carbon tetrachloride on a Fe--Fe2O3 column. The product was purified by using reversed phase, high-performance liquid chromatography (RP-HPLC) and the radioactive fraction containing S-(-)[C]CGP-12177 was collected and evaporated to dryness. S-(-)[C]CGP-12177 dissolved in physiological saline was sterilized through a 0.22 microm membrane filter. The radiochemical purity and the mass of the compound were determined with RP-HPLC. The residual organic solvents were determined with GC. Tests for sterility and the presence of bacterial endotoxins were also performed.
Results: S-(-)[C]CGP-12177 for intravenous injection was prepared in 25 min after the end of bombardment with a yield of 1.5+/-0.2 GBq. Specific activity was found to be 385.4+/-133.0 GBq/ micromol at the end of synthesis (EOS) (n=3). Radiochemical purity was found to be more than 99%. Toluene was not detected in the solution. The ethanol concentration was determined to be 60.3+/-52.5 ppm. Tests for sterility and bacterial endotoxins showed negative results.
Conclusion: We successfully prepared S-(-)[C]CGP-12177 formulated for intravenous injection with high purity and high specific activity, which is feasible for the clinical evaluation of beta-adrenoreceptors[2].
|
| Cell Assay |
Measuring the influence of unlabeled ligands on the BODIPY-TMR-CGP dissociation rate [1]
Live cell fluorescence imaging using 3 nM BODIPY-TMR-CGP was performed on the Zeiss LSM710 laser scanning confocal microscope using a Zeiss Plan-Neofluar ×40 1.3 NA oil-immersion objective in conjunction with a perfusion system as described above for the Zeiss LSM510 laser scanning confocal microscope. For association and dissociation kinetic experiments using 3 nM BODIPY-TMR-CGP in CHO-β1 and CHO-CS cells, the cells were exposed to imaging buffer only (30 s baseline fluorescence recording), then BODIPY-TMR-CGP (4 min association), and followed again by imaging buffer only (4 min dissociation). Influences of unlabeled ligands on the BODIPY-TMR-CGP dissociation rate in CHO-β1 cells were determined by perfusion of imaging buffer (30 s baseline read), 3 nM BODIPY-TMR-CGP (4 min association), and imaging buffer (4 min dissociation) in the absence or presence of CGP 12177 (0.01–10 µM) or propranolol (0.1–10 µM). In experiments using CHO-β1TM4 cells, the cells were first exposed to 3 nM BODIPY-TMR-CGP for 3.5 min in a 6-well plate prior to placing the coverslip into the imaging chamber to achieve a significant but low level of labeling of the receptor. Once placed onto the microscope stage, the cells were perfused with BODIPY-TMR-CGP (30 s baseline), before perfusing imaging buffer in the absence or presence of 1 µM CGP 12177 or 1 µM propranolol (dissociation). |
| References |
[1]. FASEB J. 2015 Jul;29(7):2859-71.
[2]. Nucl Med Commun. 2004 Aug;25(8):845-9. [3]. J Pharmacol Exp Ther. 2004 Apr;309(1):137-45. |
| Additional Infomation |
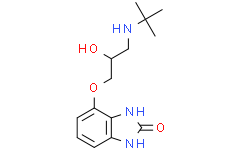
CGP 12177 is a benzimidazole that is benzimidazol-2-one substituted at position 4 by a 3-(tert-butylamino)-2-hydroxypropoxy group. It has a role as a beta-adrenergic antagonist. It is a member of benzimidazoles, an aromatic ether, a secondary amino compound and a secondary alcohol.
In summary, these data suggest that the secondary low-affinity conformation of the β1-adrenoceptor may be a consequence of negative cooperative interactions between 2 orthosteric binding conformations within a β1-adrenoceptor homodimer. This can then lead to a reduced apparent affinity of ligands for the second protomer of an already ligand-occupied (on the first protomer) dimer [see Supplemental Fig. S1 in May et al.]. The contribution of these cooperative interactions is provided by the cooperativity factor α, and the ligand affinity for the already ligand-bound receptor is described as a ratio of the ligand affinity for the unbound receptor and the cooperativity factor α (KB/α). As such, the cooperativity factor at β1-adrenoceptor dimers can be determined by taking the ratio of the apparent KD value determined for binding to the first protomer (orthosteric β1-adrenoceptor conformation 1 KD, i.e., unbound receptor) and that determined for modifying the dissociation rate of BODIPY-TMR-CGP from conformation 1 by an unlabeled ligand binding to the second protomer (allosteric β1-adrenoceptor conformation 2 KD, i.e., ligand-bound receptor). Negative cooperativity that leads to an increase in the apparent dissociation observed is reflected in a cooperativity factor smaller than unity. Indeed, the cooperativity factors (α) for CGP 12177 and propranolol were estimated to be 0.015 and 0.010, respectively, using the binding affinities for the orthosteric β1-adrenoceptor conformation determined in Gherbi et al. A mechanistic framework for the secondary β1-adrenoceptor conformation based on homodimer formation opens up new insights into the role of dimerization in altering the molecular pharmacology of GPCRs. [1] |
| Molecular Formula |
C14H22CLN3O3
|
|---|---|
| Molecular Weight |
315.8
|
| Exact Mass |
279.158
|
| Elemental Analysis |
C, 60.20; H, 7.58; N, 15.04; O, 17.18
|
| CAS # |
81047-99-6
|
| Related CAS # |
81047-99-6;81047-99-6 (HCl);
|
| PubChem CID |
2687
|
| Appearance |
Typically exists as solid at room temperature
|
| Density |
1.179g/cm3
|
| Boiling Point |
376.3ºC at 760 mmHg
|
| Flash Point |
181.4ºC
|
| Index of Refraction |
1.55
|
| LogP |
1.374
|
| Hydrogen Bond Donor Count |
4
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
6
|
| Heavy Atom Count |
20
|
| Complexity |
343
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
CC(C)(C)NCC(COC1=CC=CC2=C1NC(=O)N2)O
|
| InChi Key |
UMQUQWCJKFOUGV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19)
|
| Chemical Name |
2H-Benzimidazol-2-one, 4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)-1,3-dihydro-
|
| Synonyms |
CGP-12177; CGP12177; Cgp 12177; 81047-99-6; Cgp-12177; Cgp 12177A; 4-(3-tert-Butylamino-2-hydroxypropoxy)benzimidazol-2-one; 4-[3-(tert-butylamino)-2-hydroxypropoxy]-1,3-dihydrobenzimidazol-2-one; Tbhpbo; CHEBI:73288; CGP 12177
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1666 mL | 15.8328 mL | 31.6656 mL | |
| 5 mM | 0.6333 mL | 3.1666 mL | 6.3331 mL | |
| 10 mM | 0.3167 mL | 1.5833 mL | 3.1666 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.