
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
Purity: ≥98%
GSK429286A (also known as RHO15; GSK 429286A; RHO-15; GSK-429286A) is a potent, orally bioactive, and selective inhibitor of ROCK1/2 with antihypertensive effects. It inhibits ROCK1/2 with IC50s of 14 nM and 63 nM, respectively. It reverses adrenalin-induced contraction of the rat aortic ring (IC50 = 190 nM) and causes a dose-dependent decrease in mean arterial blood pressure in spontaneous hypertensive rats. It is shown that GSK429286A treatment (10 μM) increased MYPT phosphrylation at Thr850 via inhibiting ROCK which mediated this phosphorylation process.
| ln Vitro |
Under these circumstances, the only other kinase examined that is appreciably inhibited is MSK1, whose activity is lowered ~5-fold. GSK429286A at 1 μM lowers ROCK2 activity by nearly 20 times. According to the kinase-specificity panel, GSK429286A is a more selective ROCK2 inhibitor than the commonly used ROCK inhibitor Y-27632. It also does not appreciably inhibit LRRK2 even at dosages up to 30 μM (500-fold greater than the IC50 of ROCK2 inhibition). RSK and p70S6K are both mildly inhibited by GSK429286A, with IC50 values of 0.78 μM and 1.94 μM, respectively. Rat aortic ring dilatation is markedly inhibited by GSK429286A, with an IC50 of 190 nM[2].
|
||
|---|---|---|---|
| ln Vivo |
In male Sprague-Dawley rats, GSK429286A has an oral bioavailability of 61%. When given orally to spontaneously hypertensive rats (SHRs) at single doses ranging from 3 to 30 mg/kg, GSK429286A significantly lowers mean arterial pressure in a dose-dependent manner. The highest reduction of 50 mmHg occurs approximately two hours after treatment at a dose of 30 mg/kg[1].
|
||
| Animal Protocol |
|
||
| References | |||
| Additional Infomation |
N-(6-fluoro-1H-indazol-5-yl)-6-methyl-2-oxo-4-[4-(trifluoromethyl)phenyl]-3,4-dihydro-1H-pyridine-5-carboxamide is a member of (trifluoromethyl)benzenes.
|
| Molecular Formula |
C21H16F4N4O2
|
|
|---|---|---|
| Molecular Weight |
432.37
|
|
| Exact Mass |
432.12
|
|
| CAS # |
864082-47-3
|
|
| Related CAS # |
|
|
| PubChem CID |
11373846
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.5±0.1 g/cm3
|
|
| Boiling Point |
688.4±55.0 °C at 760 mmHg
|
|
| Flash Point |
370.2±31.5 °C
|
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
|
| Index of Refraction |
1.627
|
|
| LogP |
3.97
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
7
|
|
| Rotatable Bond Count |
3
|
|
| Heavy Atom Count |
31
|
|
| Complexity |
748
|
|
| Defined Atom Stereocenter Count |
0
|
|
| InChi Key |
OLIIUAHHAZEXEX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H16F4N4O2/c1-10-19(20(31)28-17-6-12-9-26-29-16(12)8-15(17)22)14(7-18(30)27-10)11-2-4-13(5-3-11)21(23,24)25/h2-6,8-9,14H,7H2,1H3,(H,26,29)(H,27,30)(H,28,31)
|
|
| Chemical Name |
N-(6-fluoro-1H-indazol-5-yl)-6-methyl-2-oxo-4-[4-(trifluoromethyl)phenyl]-3,4-dihydro-1H-pyridine-5-carboxamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.81 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.81 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (4.81 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 15% Captisol+citrate vehicle:15 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3128 mL | 11.5642 mL | 23.1283 mL | |
| 5 mM | 0.4626 mL | 2.3128 mL | 4.6257 mL | |
| 10 mM | 0.2313 mL | 1.1564 mL | 2.3128 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
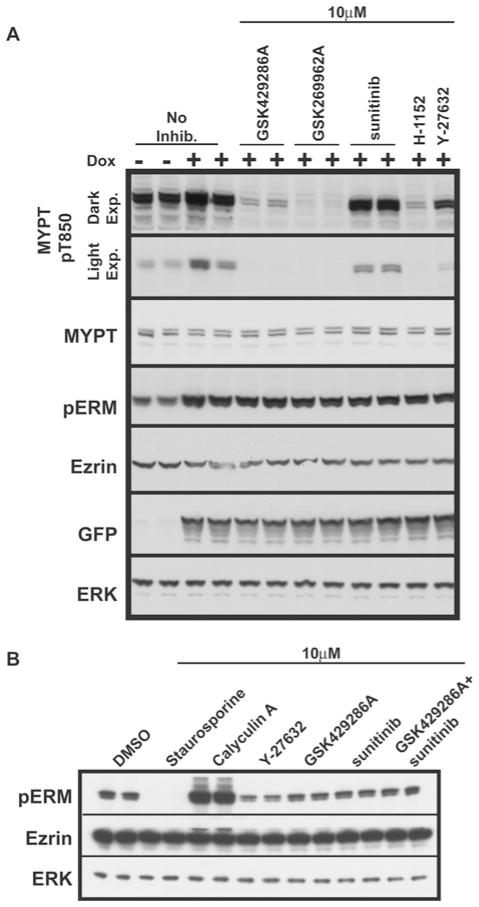 esting the efficacy of LRRK2 and ROCK inhibitorsin vivo. |
|---|
Characterization of ROCK inhibitors as LRRK2 inhibitors.Biochem J.2009 Oct 23;424(1):47-60. |
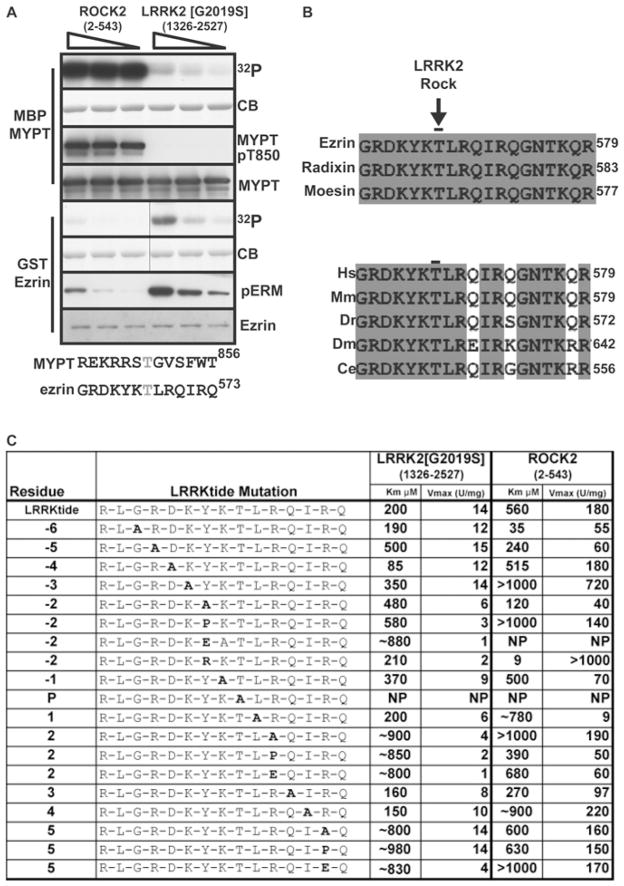 Comparison of ROCK substrates as substrates for LRRK2.Biochem J.2009 Oct 23;424(1):47-60. |