
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
MG-132, a peptide aldehyde, is a novel, specific, potent, reversible, and cell-permeable proteasome inhibitor with an IC50 of 100 nM in a cell-free assay. It also has an IC50 of 1.2 μM for calpain inhibition.
| Targets |
Proteasome (IC50 = 100 nM); Calpain (IC50 = 1.2 μM)
|
|---|---|
| ln Vitro |
MG-132 exhibits over 1000 times greater activity than ZLLal in suppressing the 20S proteasome's ZLLL-MCA-degrading activity, with an IC50 of 100 nM as opposed to 110 μM. With an IC50 of 1.2 μM, MG-132 also inhibits calpain. At an ideal concentration of 20 nM, MG-132 exhibits 500 times greater potency than ZLLal in inducing neurite outgrowth in PC12 cells.[1] MG-132 (10 μM) inhibits the proteasome-mediated degradation of IκBα in A549 cells, thereby potently inhibiting TNF-α-induced NF-κB activation, IL-8 gene transcription, and IL-8 protein release.[2] MG-132 treatment strongly inhibits 26S proteasome, thereby inducing p53-dependent apoptosis in KIM-2 cells.[3] Multiple myeloma cells (U266 and OPM-2) are more susceptible to MG-132-induced apoptosis than BzLLLCOCHO, but MG-132 treatment results in weak inhibition of the chymotrypsinlike (CT-L) and peptidylglutamyl peptide hydrolysing (PGPH) activities of the 26S proteasome, in contrast to BzLLLCOCHO or PS-341.[4] By activating AP-1 family members c-Fos and c-Jun, which in turn repress the antiapoptotic molecule c-FLIP(L), MG-132 (1 μM) sensitizes TRAIL-resistant prostate cancer cells.[5] In the PC3 and DU145 androgen-independent prostate cancer (AIPCa) cell lines, MG-132 dramatically increases inositol hexakisphosphate's (IP6) capacity to lower cellular metabolic activity.[6]
|
| ln Vivo |
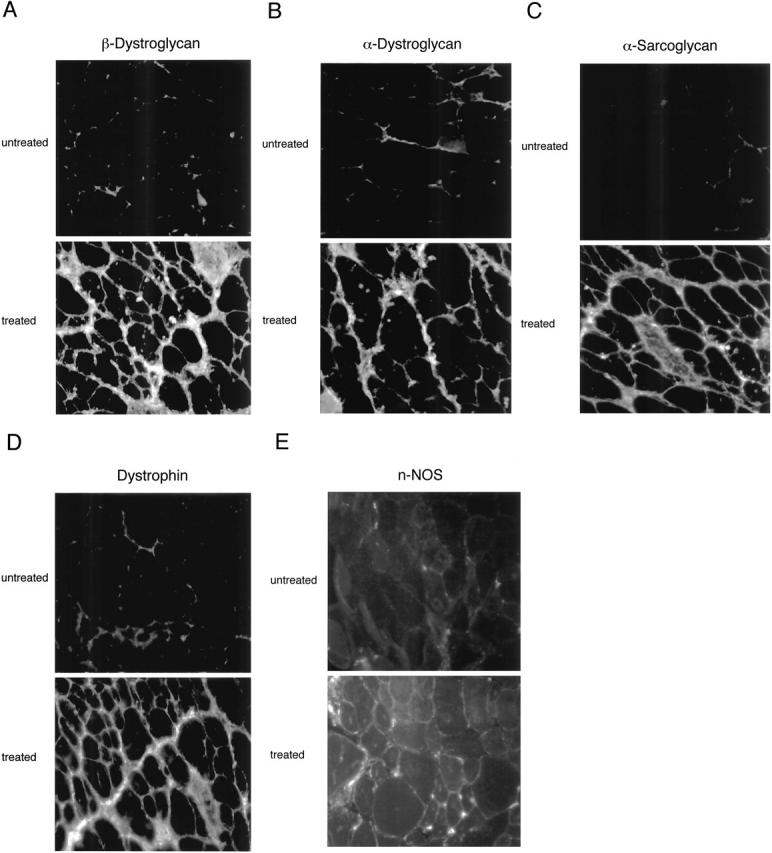
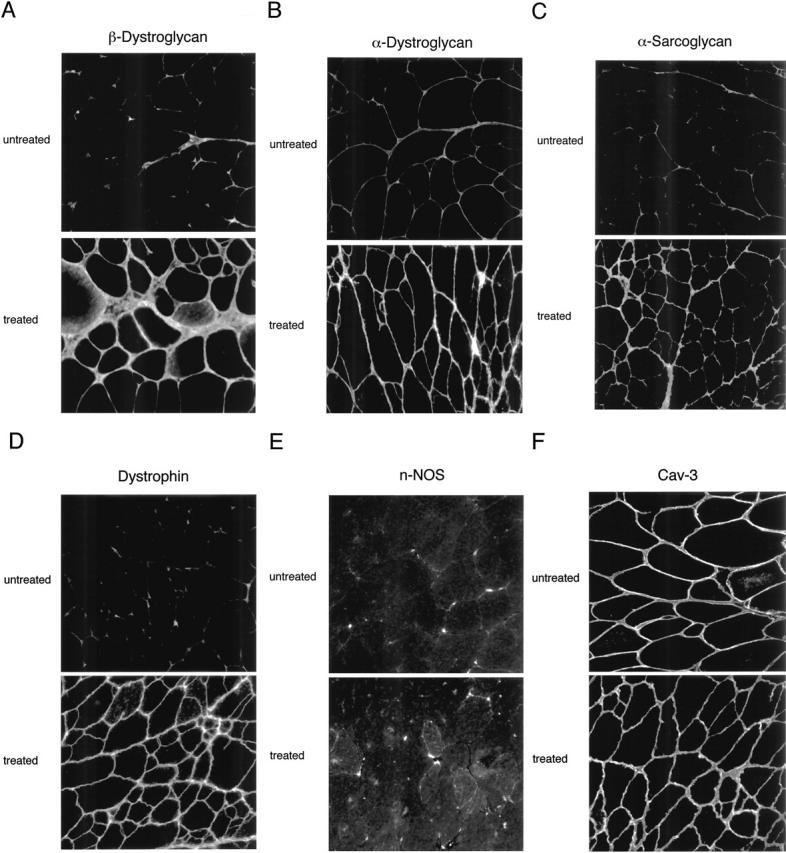
In skeletal muscle fibers from mdx mice, MG-132 administration efficiently restores the expression levels and plasma membrane localization of dystrophin, β-dystroglycan, α-bdystroglycan, and α-sarcoglycan, minimizes damage to muscle membranes, and improves the histopathological symptoms of muscular dystrophy.[8] By downregulating the muscle-specific ubiquitin ligases atrogin-1/MAFbx and MuRF-1 mRNA, MG-132 treatment dramatically reduces immobilization-induced skeletal muscle atrophy in mice.[8]
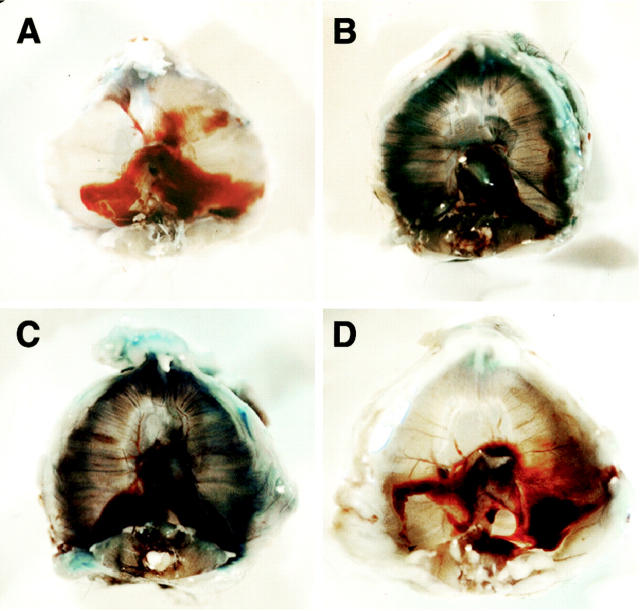
Dystrophin, the protein product of the Duchenne muscular dystrophy (DMD) gene, is absent in the skeletal muscle of DMD patients and mdx mice. At the plasma membrane of skeletal muscle fibers, dystrophin associates with a multimeric protein complex, termed the dystrophin-glycoprotein complex (DGC). Protein members of this complex are normally absent or greatly reduced in dystrophin-deficient skeletal muscle fibers, and are thought to undergo degradation through an unknown pathway. As such, we reasoned that inhibition of the proteasomal degradation pathway might rescue the expression and subcellular localization of dystrophin-associated proteins. To test this hypothesis, we treated mdx mice with the well-characterized proteasomal inhibitor MG-132. First, we locally injected MG-132 into the gastrocnemius muscle, and observed the outcome after 24 hours. Next, we performed systemic treatment using an osmotic pump that allowed us to deliver different concentrations of the proteasomal inhibitor, over an 8-day period. By immunofluorescence and Western blot analysis, we show that administration of the proteasomal inhibitor MG-132 effectively rescues the expression levels and plasma membrane localization of dystrophin, beta-dystroglycan, alpha-dystroglycan, and alpha-sarcoglycan in skeletal muscle fibers from mdx mice. Furthermore, we show that systemic treatment with the proteasomal inhibitor 1) reduces muscle membrane damage, as revealed by vital staining (with Evans blue dye) of the diaphragm and gastrocnemius muscle isolated from treated mdx mice, and 2) ameliorates the histopathological signs of muscular dystrophy, as judged by hematoxylin and eosin staining of muscle biopsies taken from treated mdx mice. Thus, the current study opens new and important avenues in our understanding of the pathogenesis of DMD. Most importantly, these new findings may have clinical implications for the pharmacological treatment of patients with DMD. [7] In the present study, we showed that the proteasome inhibitor MG132 significantly inhibited IκBα degradation thus preventing NFκB activation in vitro. MG132 preserved muscle and myofiber cross-sectional area by downregulating the muscle-specific ubiquitin ligases atrogin-1/MAFbx and MuRF-1 mRNA in vivo. This effect resulted in a diminished rehabilitation period. Conclusion: These finding demonstrate that proteasome inhibitors show potential for the development of pharmacological therapies to prevent muscle atrophy and thus favor muscle rehabilitation [8]. Treatment of recurrent or advanced cervical cancer is still limited, and new therapeutic choices are needed for improving prognosis and quality of life of patients. Because human papilloma virus (HPV) infection is critical in cervical carcinogenesis, with the E6 and E7 oncogenes of HPV degrading tumor suppressor proteins through the ubiquitin proteasome system, the inhibition of the ubiquitin proteasome system appears to be an ideal target to suppress the growth of cervical tumors. Herein, we focused on the ubiquitin proteasome inhibitor MG132 (carbobenzoxy-Leu-Leu-leucinal) as an anticancer agent against cervical cancer cells, and physically incorporated it into micellar nanomedicines for achieving selective delivery to solid tumors and improving its in vivo efficacy. These MG132-loaded polymeric micelles (MG132/m) showed strong tumor inhibitory in vivo effect against HPV-positive tumors from HeLa and CaSki cells, and even in HPV-negative tumors from C33A cells. Repeated injection of MG132/m showed no significant toxicity to mice under analysis by weight change or histopathology. Moreover, the tumors treated with MG132/m showed higher levels of tumor suppressing proteins, hScrib and p53, as well as apoptotic degree, than tumors treated with free MG132. This enhanced efficacy of MG132/m was attributed to their prolonged circulation in the bloodstream, which allowed their gradual extravasation and penetration within the tumor tissue, as determined by intravital microscopy. These results support the use of MG132 incorporated into polymeric micelles as a safe and effective therapeutic strategy against cervical tumors[10]. |
| Enzyme Assay |
MG-132, 20S proteasome, pH 7.0, 0.1 M Tris-acetate, and 25 μM substrate dissolved in dimethyl sulfoxide in a final volume of 1 mL make up the reaction mixture for the 20S proteasome inhibitory assay. 0.1 mL of 10% SDS and 0.9 mL of 0.1M Tris acetate, pH 9.0, are added to stop the reaction after it has been incubated at 37 °C for 15 minutes. The reaction products' fluorescence is measured. Different concentrations of MG-132 are added to the assay mixture in order to calculate the IC50 against 20S proteasome.
The 26S proteasome is a multicatalytic protease responsible for regulated intracellular protein degradation. Its function is mediated by three main catalytic activities: (a) chymotrypsin-like (CT-L), (b) trypsin-like, and (c) peptidylglutamyl peptide hydrolysing (PGPH). Proteasome inhibition is an emerging therapy for many cancers and is a novel treatment for multiple myeloma. Here, we profile the contributions of the three catalytic activities in multiple myeloma cell lines and compare the specificity and cytotoxicity of the novel proteasome inhibitor BzLLLCOCHO and inhibitors PS-341 (Velcade, bortezomib) and MG-132. Using fluorogenic substrates and an active site-directed probe specific for proteasome catalytic subunits, we show differential subunit specificity for each of the inhibitors. Addition of BzLLLCOCHO strongly inhibited all three catalytic activities, treatment with PS-341 completely inhibited CT-L and PGPH activities, and treatment with MG-132 resulted in weak inhibition of the CT-L and PGPH activities. Multiple myeloma cells were more sensitive to induction of apoptosis by PS-341 and MG-132 than BzLLLCOCHO. This study emphasizes the need for further investigation of the effects of these compounds on gene and protein expression in the cell to allow for the development of more specific and targeted inhibitors [4]. |
| Cell Assay |
MG-132 is added to cells at different concentrations for 24, and 48 hours. Centrifugation is used to collect the supernatant and monolayer cells, which are then preserved in 70% ethanol in PBS before being stained with acridine orange. Acridine orange (5 mg/mL in PBS) and equal volumes of cells are combined on a microscope slide, and fluorescence microscopy is used to examine the mixture. Cells are collected by centrifugation and stained with propidium iodide and annexin V for annexin V analysis. Propidium iodide (5 mg/mL) staining is done after rehydrating cells in PBS at room temperature for ten minutes in order to analyze the cell cycle. Utilizing a Coulter Epics XL flow cytometer, every sample is examined.
Cell Viability Assay[1] Cell Types: C6 glioma cells Tested Concentrations: 10, 20, 30, 40 μM Incubation Duration: 24 hrs (hours) Experimental Results: Dramatically decreased the viability of C6 glioma cells beginning at 6 h in both time- and concentration-dependent manners and showed the IC50 of 18.5 μM at 24 hours. Cell Viability Assay[1] Cell Types: A549 cells Tested Concentrations: 10 μM Incubation Duration: 1 hrs (hours) Experimental Results: Reversed the effects of TNF-α on IκB degradation and resulted in a reversal of TNF-α-induced NF-κB activation. |
| Animal Protocol |
Animal/Disease Models: Female athymic nude mice bearing EC9706 xenograft (5- to 6-weeks old) [10]
Doses: 10 mg/kg Route of Administration: i.p.; daily for 25 days starting 5 days after inoculation of EC9706 tumors Experimental Results: Dramatically suppressed tumor growth of the EC9706 xenograft without apparrent toxicity to mice. Animal/Disease Models: Female C.B-17/lcr-scid/scidJcl mice bearing HeLa tumors (5- to 6-weeks old) [10] Doses: 10 mg/kg Route of Administration: Intravenous/i.v. injection; twice a week for 4 weeks Experimental Results: The growth inhibition rates in HeLa tumors was 49% compared to the control. Dramatically suppressed tumor growth of HeLa tumors with a TGI of 49%. Male mdx (C57BL/10ScSn DMD mdx) mice ~10 μg/kg/day Injection |
| References |
[1]. J Biochem. 1996 Mar;119(3):572-6. [2]. Am J Respir Cell Mol Biol. 1998 Aug;19(2):259-68. [3]. Cell Death Differ. 2001 Mar;8(3):210-8. [4]. Cancer Res. 2006 Jun 15;66(12):6379-86. [5]. J Med ChemCancer Res. 2007 Mar 1;67(5):2247-55. [6]. Br J Cancer. 2008 Nov 18;99(10):1613-22. [7]. Am J Pathol. 2003 Oct;163(4):1663-75. [8]. BMC Musculoskelet Disord. 2011 Aug 15:12:185. |
| Additional Infomation |
N-benzyloxycarbonyl-L-leucyl-L-leucyl-L-leucinal is a tripeptide that is L-leucyl-L-leucyl-L-leucine in which the C-terminal carboxy group has been reduced to the corresponding aldehyde and the N-terminal amino group is protected as its benzyloxycarbonyl derivative. It has a role as a proteasome inhibitor. It is a tripeptide, an amino aldehyde and a carbamate ester.
Z-Leu-leu-leu-al has been reported in Tricholoma pardinum, Glycyrrhiza glabra, and Glycyrrhiza inflata with data available. To explore membrane-permeable synthetic inhibitors that discriminate between endogenous calpain and proteasome in cells, we examined the inhibition of profiles against calpain and proteasome in vitro and in vivo of peptidyl aldehydes possessing di-leucine and tri-leucine. The tripeptide aldehyde benzyloxycarbonyl-leucyl-leucinal (ZLLLal) strongly inhibited calpain and proteasome activities in vitro. The concentration required for 50% inhibition (IC50) of the casein-degrading activity of calpain was 1.25 microM, and the IC50s for the succinyl-leucyl-leucyl-valyl-tyrosine-4-methylcoumaryl-7-amide (Suc-LLVY-MCA)- and benzyloxycarbonyl-leucyl-leucyl-leucine-4-methylcoumaryl -7-amide (ZLLL-MCA)-degrading activities of proteasome were 850 and 100 nM, respectively. On the other hand, the synthetic dipeptide aldehyde benzyloxycarbonyl-leucyl-leucinal (ZLLal) strongly inhibited the casein degrading activity of calpain (IC50 1.20 microM), but the inhibition of proteasome was weak (IC50S for SucLLVY-MCA- and ZLLL-MCA-degrading activities were 120 and 110 microM, respectively). Thus, while calpain was inhibited by similar concentrations of ZLLal and ZLLLal, the inhibitory potencies of ZLLLal against the ZLLL-MCA- and Suc-LLVY-MCA-degrading activities in proteasome were 1,100 and 140 times stronger than those of ZLLal, respectively. To evaluate the effectiveness of these inhibitors on intracellular proteasome, the induction of neurite outgrowth in PC12 cells caused by proteasome inhibition was examined. ZLLLal and ZLLal initiated neurite outgrowth with optimal concentrations of 20 nM and 10 microM, respectively, again showing a big difference in the effective concentrations for the proteasome inhibition as in vitro. As for the effect on intracellular calpain, the concentration of ZLLLal and ZLLal required for the inhibition of the autolytic activation of calpain in rabbit erythrocytes were 100 and 100 microM or more, respectively. The almost equal inhibitory potencies of ZLLLal and ZLLal were in agreement with the inhibition of calpain in vitro. These differential effects of inhibitors against calpain and proteasome are potentially useful for identifying the functions of calpain and proteasome in cell physiology and pathology. [1] The working hypothesis of the studies described herein was that inhibition of proteasome-mediated IkappaB degradation would inhibit TNF-alpha-induced nuclear factor-kappaB (NF-kappaB) activation, interleukin-8 (IL-8) gene transcription, and IL-8 protein release in A549 cells. Mutational analysis of the 5' flanking region of the IL-8 gene confirmed that an intact NF-kappaB site is necessary for TNF-alpha-induced IL-8 gene transcription. The addition of TNF-alpha to A549 cells resulted in rapid loss of IkappaB from the cytoplasm of cells, associated with a corresponding increase in NF-kappaB-binding activity in nuclear extracts from the cells. However, pretreatment of the cells with the proteasome inhibitor N-cbz-Leu-Leu-leucinal (MG-132, 10 microM) reversed the effects of TNF-alpha on IL-8 release from A549 cells (as determined with an enzyme-linked immunosorbent assay [ELISA]) and on IL-8 gene transcription (as determined with reporter-gene assays). MG-132 reversed the effects of TNF-alpha on IkappaB degradation as determined by Western blot analysis. IkappaB phosphorylation and ubiquination were not altered by MG-132, which implies that the effects of MG-132 were secondary to proteasome inhibition. MG-132 also reversed the increase in NF-kappaB binding in nuclear extracts from TNF-alpha-treated cells. These studies show that inhibition of proteasome-mediated IkappaB degradation results in inhibition of TNF-alpha induced IL-8 production in A549 cells by limiting NF-kappaB-mediated gene transcription.[2] We have examined the effects of inhibition of the 26S proteasome in a murine mammary cell line, KIM-2 cells using the peptide aldehyde inhibitor MG132. These studies have demonstrated a clear requirement for proteasome function in cell viability. Induction of apoptosis was observed following MG132 treatment in KIM-2 cells and this death was shown to be dependent on the cell actively traversing the cell cycle. KIM-2 cells were generated using a temperature sensitive T-antigen (Tag) and studies at the permissive temperature (33 degrees C) have shown that a Tag binding protein was essential for this apoptotic response. Studies in two additional cell lines, HC11, which is a mammary epithelial cell line carrying mutant p53 alleles and p53 null ES cells suggest that p53 is actively required for the apoptosis induced as a consequence of proteasome inhibition. These results suggest a pivotal role for the 26S proteasome degradation pathway in progression through the cell cycle in proliferating cells. [3] Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer agent because it induces apoptosis in cancer cells but not in normal cells. Unfortunately, some cancer cells develop resistance to TRAIL-induced apoptosis. Therefore, it is clinically relevant to determine the molecular mechanisms that differentiate between TRAIL-sensitive and TRAIL-resistant tumors. Previously, we have shown that the antiapoptotic molecule cellular-FLICE-inhibitory protein long isoform [c-FLIP(L)] is necessary and sufficient to maintain resistance to TRAIL-induced apoptosis. We have found that c-FLIP(L) is transcriptionally regulated by the activator protein-1 (AP-1) family member protein c-Fos. Here, we report that MG-132, a small-molecule inhibitor of the proteasome, sensitizes TRAIL-resistant prostate cancer cells by inducing c-Fos and repressing c-FLIP(L). c-Fos, which is activated by MG-132, negatively regulates c-FLIP(L) by direct binding to the putative promoter region of the c-FLIP(L) gene. In addition to activating c-Fos, MG-132 activates another AP-1 family member, c-Jun. We show that c-Fos heterodimerizes with c-Jun to repress transcription of c-FLIP(L). Therefore, MG-132 sensitizes TRAIL-resistant prostate cancer cells by activating the AP-1 family members c-Fos and c-Jun, which, in turn, repress the antiapoptotic molecule c-FLIP(L).[5] Effective treatments for androgen-independent prostate cancer (AIPCa) are lacking. To address this, emerging therapeutics such as proteasome inhibitors are currently undergoing clinical trials. Inositol hexakisphosphate (IP6) is an orally non-toxic phytochemical that exhibits antitumour activity against several types of cancer including PCa. We have previously shown that treatment of PC3 cells with IP6 induces the transcription of a subset of nuclear factor-kappaB (NF-kappaB)-responsive and pro-apoptotic BCL-2 family genes. In this study, we report that although NF-kappaB subunits p50/p65 translocate to the nucleus of PC3 cells in response to IP6, inhibition of NF-kappaB-mediated transcription using non-degradable inhibitor of kappaB (IkappaB)-alpha does not modulate IP6 sensitivity. Treatment with IP6 also leads to increased protein levels of PUMA, BIK/NBK and NOXA between 4 and 8 h of treatment and decreased levels of MCL-1 and BCL-2 after 24 h. Although blocking transcription using actinomycin D does not modulate PC3 cell sensitivity to IP6, inhibition of protein translation using cycloheximide has a significant protective effect. In contrast, blocking proteasome-mediated protein degradation using MG-132 significantly enhances the ability of IP6 to reduce cellular metabolic activity in both PC3 and DU145 AIPCa cell lines. This effect of combined treatment on mitochondrial depolarisation is particularly striking and is also reproduced by another proteasome inhibitor (ALLN). The enhanced effect of combined MG132/IP6 treatment is almost completely inhibited by cycloheximide and correlates with changes in BCL-2 family protein levels. Altogether these results suggest a role for BCL-2 family proteins in mediating the combined effect of IP6 and proteasome inhibitors and warrant further pre-clinical studies for the treatment of AIPCa.[6] MG-132 is a tripeptide aldehyde (Z-l-leu-l-leu-l-leu-H, 2) proteasome inhibitor that exerts antitumor activity and enhances cytostatic/cytotoxic effects of chemo- and radiotherapy. Because of a troublesome synthesis of tripeptides with a non-natural configuration and modified side chains of amino acids, only two stereoisomers of MG-132 have been reported. Here, we propose a new approach to the synthesis of tripeptide aldehydes based on the Ugi reaction. Chiral, enantiomerically stable 2-isocyano-4-methylpentyl acetates were used as substrates for Ugi reaction resulting in a formation of tripeptide skeletons. Further functionalization of the obtained products led to a synthesis of tripeptide aldehydes. All stereoisomers of MG-132 were synthesized and studied as potential inhibitors of chymotrypsin-like, trypsin-like, and peptidylglutamyl peptide hydrolyzing activities of proteasome. These studies demonstrated the influence of absolute configuration of chiral aldehydes on the cytostatic/cytotoxic effects of the synthesized compounds and revealed that only (S,R,S)-(-)-2 stereoisomer is a more potent proteasome inhibitor than MG-132. [9] |
| Molecular Formula |
C26H41N3O5
|
|---|---|
| Molecular Weight |
475.62
|
| Exact Mass |
475.304
|
| Elemental Analysis |
C, 65.66; H, 8.69; N, 8.83; O, 16.82
|
| CAS # |
133407-82-6
|
| Related CAS # |
(R)-MG-132;1211877-36-9;MG-132 (negative control)
|
| PubChem CID |
462382
|
| Sequence |
Z-Leu-Leu-Leu-al;
N-benzoxycarbonyl-L-leucyl-L-leucyl-L-leucinal
|
| SequenceShortening |
Cbz-Leu-Leu-Leu-al; LLL
|
| Appearance |
White to yellow solid powder
|
| Density |
1.1±0.1 g/cm3
|
| Boiling Point |
682.0±55.0 °C at 760 mmHg
|
| Melting Point |
80-84℃ (DEC.)
|
| Flash Point |
366.3±31.5 °C
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
| Index of Refraction |
1.506
|
| LogP |
5.75
|
| Hydrogen Bond Donor Count |
3
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
15
|
| Heavy Atom Count |
34
|
| Complexity |
644
|
| Defined Atom Stereocenter Count |
3
|
| SMILES |
O=C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C(=O)OC([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])=O)N([H])[C@]([H])(C([H])=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
TZYWCYJVHRLUCT-VABKMULXSA-N
|
| InChi Code |
InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1
|
| Chemical Name |
benzyl N-[(2S)-4-methyl-1-[[(2S)-4-methyl-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate
|
| Synonyms |
MG-132; MG 132; MG132; MGI132; MGI 132; Z-Leu-leu-leu-al; Zlllal; Z-Leu-leu-leucinal; Z-LLL-CHO; MGI-132
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.67 mg/mL (3.51 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 1.67 mg/mL (3.51 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1.67 mg/mL (3.51 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 4% DMSO+30% PEG 300+20% propylene glycol+ddH2O: 2 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1025 mL | 10.5126 mL | 21.0252 mL | |
| 5 mM | 0.4205 mL | 2.1025 mL | 4.2050 mL | |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1025 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
 |
 |
|
|---|
 |