
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
Purity: =99.88%
Nintedanib esylate (formerly also known as BIBF 1120 esylate; BIBF-1120 esylate; Vargatef), the esylate salt of nintedanib, is a novel, orally bioavailable, potent triple angiokinase inhibitor that has gained FDA approval in 2014 for the treatment of idiopathic pulmonary fibrosis (IPF). In cell-free experiments, it inhibits several kinases, including VEGFR1/2/3, FGFR1/2/3, and PDGFRα/β, with IC50 values of 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM, and 59 nM/65 nM. With its specific binding to and inhibition of VEGFR, FGFR, and PDGFR tyrosine kinases, intedanib has the potential to cause endothelial cell apoptosis, decrease tumor vasculature, and inhibit tumor cell migration and proliferation. On November 15, 2014, the FDA approved netedanib to treat idiopathic pulmonary fibrosis (IPF).
| Targets |
VEGFR1 (IC50 = 34 nM); VEGFR2 (IC50 = 13 nM); VEGFR3 (IC50 = 13 nM); FGFR1 (IC50 = 69 nM); FGFR2 (IC50 = 37 nM); FGFR3 (IC50 = 108 nM); PDGFRα (IC50 = 59 nM); VEGFR1 (IC50 = 34 nM); PDGFRβ (IC50 = 65 nM)
|
|---|---|
| ln Vitro |
Nintedanib (BIBF 1120) attaches itself to the ATP-binding site of the kinase domain, which is located in the cleft between the amino and carboxy terminal lobes. With an EC50 of 79 nM in cell assays, neintedanib (BIBF 1120) inhibits the proliferation of PDGF-BB stimulated BRPs. After stimulation with 5% serum plus PDGF-BB, neintedanib (BIBF 1120) (100 nM) inhibits MAPK activation. In cultures of human vascular smooth muscle cells (HUASMC), neintedanib (BIBF 1120) inhibits PDGF-BB stimulated proliferation with an EC50 of 69 nM[1].
|
| ln Vivo |
Nintedanib (BIBF 1120) 25–100 mg/kg daily p.o. is very active in all tumor models, including a syngeneic rat tumor model and human tumor xenografts growing in nude mice. This is demonstrated by the tumor's perfusion on magnetic resonance imaging after three days, its decreased vessel integrity and density after five days, and its significant growth inhibition[1]. Orally administered nitedanib (BIBF 1120) is well tolerated and shows encouraging efficacy in in vivo tumor models[2].
|
| Enzyme Assay |
The pFastBac clone containing the cytoplasmic tyrosine kinase domain of VEGFR2 (residues 797–1355 based on the sequence deposited in databank SWISS-PROT P35968) is fused to GST and extracted. The assay of enzyme activity is conducted in 25% DMSO with or without serial dilutions of BIBF1120. There are internal controls on every microtiter plate, including blank, maximum reaction, and historical reference compound. On a rotating shaker, all incubations are carried out at room temperature. One hour is spent preincubating 10 μL of diluted kinase (0.8 μg/mL VEGFR2, 10 mM Tris pH 7.5, 2 mM EDTA, and 2 mg/mL BSA) with 10 μL of each BIBF1120 dilution. Addition of 30 μL of substrate mix containing 13.3 mM Mg-acetate, 6.2.4 mM Tris pH 7.5, 2.7 mM DTT, 5.3 mM MnCl2, 0.42 mM ATP, 0.83 mg/mL Poly-Glu-Tyr(4:1), and 1.7 μg/mL Poly-Glu-Tyr(4:1)-biotin initiates the reaction, which is then incubated for one hour. 90 μL of the reaction mix is placed on a streptavidin plate and incubated for one to two hours. The reaction is stopped by adding 50 μL of 250 mM EDTA, 20 mM HEPES, and pH 7.4. PY20 is added (recommended dilution 1:2000 of 0.5 mg/mL labeled antibody in DELFIA assay buffer) following three PBS washes with the EU-labeled antibody. Three DELFIA washing buffer washes are used to get rid of extra detection antibody. The DELFIA enhancement solution (100 μL) is then incubated in each well 10 minutes prior to measurement on the multilabel reader.
|
| Cell Assay |
For the assay, the cell lines BRP, HUASMC, and HUVEC are employed. The cultures are supplemented with BIBF1120 two hours prior to the addition of ligands. There are cell lysates produced. Standard SDS-PAGE techniques are used for western blotting, with 50–75 μg of protein loaded per lane. Improved chemiluminescence aids in detection. Monoclonal antibodies M3807 and M8159 are used to analyze total and phosphorylated mitogen-activated protein kinase (MAPK). The monoclonal antibody for phosphorylated Akt (Ser473) is used to analyze it, while the corresponding polyclonal antibody is used to detect total Akt. While a corresponding antibody is used to detect KDR (VEGFR2) protein, monoclonal antibodies are also utilized to detect cleaved caspase-3.
|
| Animal Protocol |
For the assay, athymic NMRI-nu/nu female mice weighing between 21 and 33 grams are five to six weeks old. Following their acclimation, mice are injected with 1 to 5×106 (in 100 μL) of SKOV-3, FaDu, Caki-1, H460, HT-29, or PAC-120 cells subcutaneously into their right flank. Following their acclimation, 5×106 (in 100 μL) GS-9L cells are subcutaneously injected into the right flank of F344 Fischer rats. Blood is extracted from the retroorbital plexus of mice at predetermined intervals for pharmacokinetic analysis, and plasma is examined using high performance liquid chromatography-mass spectrometry methodology[1].
|
| References |
|
| Molecular Formula |
C33H39N5O7S
|
|---|---|
| Molecular Weight |
649.763
|
| Exact Mass |
649.25701977
|
| Elemental Analysis |
C, 61.00; H, 6.05; N, 10.78; O, 17.24; S, 4.93
|
| CAS # |
656247-18-6
|
| Related CAS # |
Nintedanib;656247-17-5
|
| Appearance |
Yellow solid powder
|
| LogP |
4.62
|
| tPSA |
160.46
|
| SMILES |
CCS(=O)(=O)O.CN1CCN(CC1)CC(=O)N(C)C2=CC=C(C=C2)N=C(C3=CC=CC=C3)C4=C(NC5=C4C=CC(=C5)C(=O)OC)O
|
| InChi Key |
ZNMRDZZRAFJOKY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H33N5O4.C2H6O3S/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38;1-2-6(3,4)5/h4-14,19,33,38H,15-18,20H2,1-3H3;2H2,1H3,(H,3,4,5)
|
| Chemical Name |
ethanesulfonic acid;methyl 2-hydroxy-3-[N-[4-[methyl-[2-(4-methylpiperazin-1-yl)acetyl]amino]phenyl]-C-phenylcarbonimidoyl]-1H-indole-6-carboxylate
|
| Synonyms |
BIBF1120; BIBF 1120 BIBF-1120; Nintedanib esylate; Nintedanib ethanesulfonate salt; Intedanib; Brand name: OFEV; Vargatef
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~41.7 mg/mL (~64.1 mM)
H2O: ~16.7 mg/mL (~25.7 mM) Ethanol: ~3.1 mg/mL (~4.7 mM) |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 5% DMSO+40% PEG 300+2% Tween 80+ddH2O: 0.25mg/mL Solubility in Formulation 5: 10 mg/mL (15.39 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 6: 20 mg/mL (30.78 mM) in 20% HP-β-CD in Saline (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5390 mL | 7.6951 mL | 15.3903 mL | |
| 5 mM | 0.3078 mL | 1.5390 mL | 3.0781 mL | |
| 10 mM | 0.1539 mL | 0.7695 mL | 1.5390 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02902484 | Active Recruiting |
Drug: Nintedanib | Cancer of Pancreas | University of Texas Southwestern Medical Center |
September 7, 2017 | Phase 1 Phase 2 |
| NCT02009579 | Active Recruiting |
Drug: Nintedanib Drug: Placebo |
Uterine Cervical Neoplasms | Belgian Gynaecological Oncology Group |
March 2014 | Phase 2 |
| NCT04559581 | Active Recruiting |
Drug: Nintedanib | Lung Diseases, Interstitial | Boehringer Ingelheim | September 28, 2020 | |
| NCT02496585 | Active Recruiting |
Drug: Nintedanib Drug: Prednisone |
Lung Cancer Lung Metastases |
Memorial Sloan Kettering Cancer Center |
July 2015 | Phase 2 |
| NCT05065190 | Active Recruiting |
Drug: nintedanib Drug: Placebo |
Lung Diseases, Interstitial | Boehringer Ingelheim | November 25, 2021 | Phase 3 |
Cancer Res. 2008 Jun 15;68(12):4774-82. |
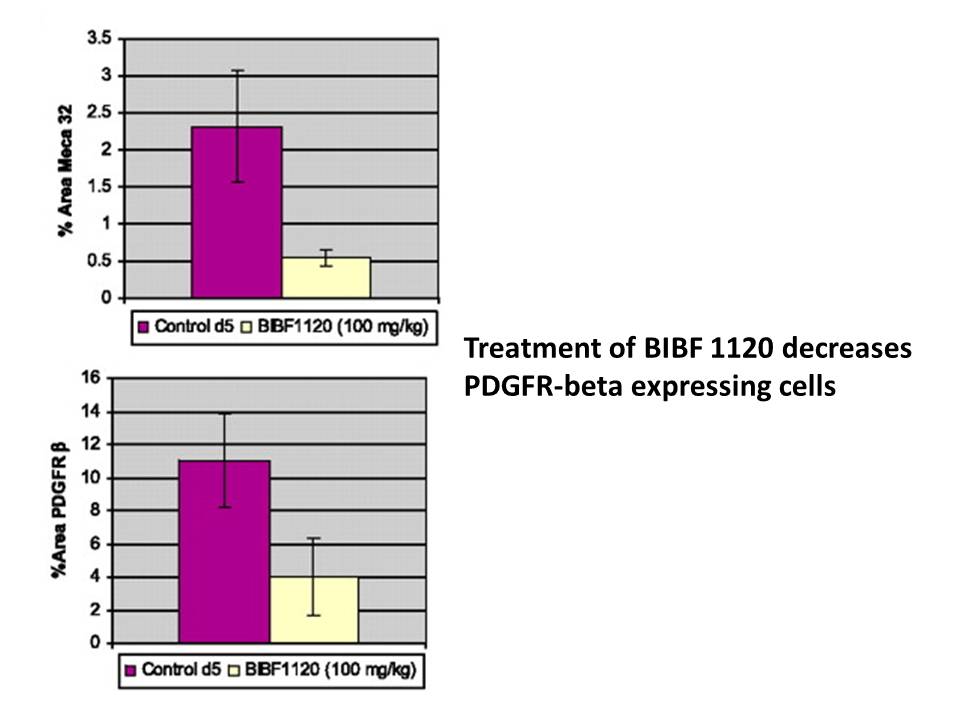 |
Cancer Res. 2008 Jun 15;68(12):4774-82. |