
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
ONO-7300243 (ONO 7300243, ONO7300243) is a novel, potent and selective antagonist of LPA1(Lysophosphatidic Acid Receptor) with an IC50 of 160 nM. ONO-7300243 was identified from a high throughout screening and subsequent structural optimizaiton. ONO-7300243 was found at a high through structural optimization that followed screening. In vivo, ONO-7300243 exhibits good efficacy. LPA is known to elicit different physiological responses via a group of G protein-coupled receptors called LPA1-6. Notably, research revealed that ONO-7300243 exhibited potency comparable to tamsulosin, a α1 adrenoceptor antagonist used clinically to treat dysuria in patients with benign prostatic hyperplasia (BPH). Rats given 30 mg/kg of 17a orally experience a decrease in intraurethral pressure.
| Targets |
LPA1 ( IC50 = 0.19-0.13 μM )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay |
The 96-well plates were seeded with 2×104 Chinese hamster ovary (CHO) cells that were stably expressing human LPA1. The cells were then cultured in the F-12 Nutrient Mixture (HAM) culture medium, which contained 10% FBS, in a CO2 incubator (37ºC, 5% CO2, 95% air) for two days. Each well was filled with load buffer (culture medium containing 5 µM Fura2-AM, 10 mM HEPES (pH 7.55), and 2.5 mM probenecid), which was then incubated for an hour in a CO2 incubator. The cells were rinsed with room-temperature assay buffer after the load buffer was removed, and then the assay buffer was added. Using a fluorescence drug screening system, the intracellular Ca2+ concentration was tracked in the LPA1 antagonist assay experiment by measuring the ratio of fluorescence intensities (f340/f380) at 500 nm. The cells were treated with lysophosphatidic acid (LPA) at a final concentration of 100 nM following the antagonist pretreatment. After treating the compounds, the peak ratio of LPA was compared to that of the control (DMSO) to determine the antagonists' inhibition rate (%). In addition, the Sigmoid Emax Model was used in a non-linear regression analysis to estimate IC50 values.
|
||
| Animal Protocol |
|
||
| References |
| Molecular Formula |
C28H31NO5
|
|
|---|---|---|
| Molecular Weight |
461.55
|
|
| Exact Mass |
461.22
|
|
| Elemental Analysis |
C, 72.86; H, 6.77; N, 3.03; O, 17.33
|
|
| CAS # |
638132-34-0
|
|
| Related CAS # |
|
|
| PubChem CID |
66775043
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.2±0.1 g/cm3
|
|
| Boiling Point |
688.1±55.0 °C at 760 mmHg
|
|
| Flash Point |
369.9±31.5 °C
|
|
| Vapour Pressure |
0.0±2.3 mmHg at 25°C
|
|
| Index of Refraction |
1.591
|
|
| LogP |
5.59
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
11
|
|
| Heavy Atom Count |
34
|
|
| Complexity |
612
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(C1C([H])=C(C(C([H])([H])[H])=C(C=1[H])OC([H])([H])[H])OC([H])([H])[H])N(C([H])([H])C1C([H])=C([H])C(C([H])([H])C(=O)O[H])=C([H])C=1[H])C([H])([H])C([H])([H])C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H]
|
|
| InChi Key |
WGABOZPQOOZAOI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C28H31NO5/c1-20-25(33-2)17-24(18-26(20)34-3)28(32)29(15-7-10-21-8-5-4-6-9-21)19-23-13-11-22(12-14-23)16-27(30)31/h4-6,8-9,11-14,17-18H,7,10,15-16,19H2,1-3H3,(H,30,31)
|
|
| Chemical Name |
2-[4-[[(3,5-dimethoxy-4-methylbenzoyl)-(3-phenylpropyl)amino]methyl]phenyl]acetic acid
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (6.50 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (6.50 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1666 mL | 10.8331 mL | 21.6661 mL | |
| 5 mM | 0.4333 mL | 2.1666 mL | 4.3332 mL | |
| 10 mM | 0.2167 mL | 1.0833 mL | 2.1666 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
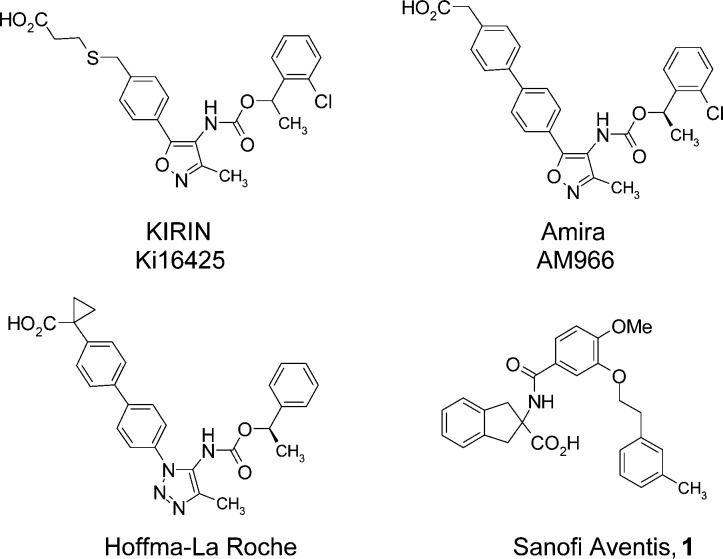 Structures of known LPA1 antagonists.
Docking results for some of our compounds using LPA1 crystal structure (PDB code 4z34). ACS Med Chem Lett.2016 Aug 19;7(10):913-918. |
|---|
In vivoefficacy ofONO-7300243(17a).ACS Med Chem Lett.2016 Aug 19;7(10):913-918. |