
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Palbociclib Isethionate (formerly known as PD0332991; PD-0332991; Ibrance), the isethionate salt of Palbociclib, is an orally bioavailable pyridopyrimidine-based inhibitor of CDK4/6 with potential antitumor activity and was approved as an anticancer drug. In cell-free experiments, it inhibits CDK4/6 with IC50s of 11 nM and 16 nM, respectively. Many tumor cells overexpress CDK4 and CDK6, and Pfizer's palbociclib is the first CDK4/6 inhibitor to be approved by the FDA as a cancer treatment in 2017. There is no evidence of any activity against PDGFR, EGFR, FGFR, CDK1/2/5, InsR, etc. In vitro, it is a strong anti-proliferative agent that induces an exclusive G1 arrest in Rb-positive tumor cells. It has been shown to cause G1 arrest in primary bone marrow cells and stop tumor growth in disseminated human myeloma xenografts.
| Targets |
Cdk4/cyclin D3 (IC50 = 9 nM); Cdk4/cyclin D1 (IC50 = 11 nM); Cdk6/cyclin D2 (IC50 = 16 nM); DYRK1A (IC50 = 2000 nM); MAPK (IC50 = 8000 nM)
|
|---|---|
| ln Vitro |
PD 0332991 shows complete CDK4/6 selectivity and negligible or no activity against other CDKs. With an IC50 of 66 nM and 63 nM, respectively, PD 0332991 effectively lowers Rb phosphorylation at Ser780 and Ser795 in MDA-MB-435 breast cancer cells. By keeping cells from going into S phase, PD 0332991 suppresses DNA replication and is a strong inhibitor of cell growth. With IC50 values ranging from 0.04-0.17 μM, PD 0332991 inhibits thymidine incorporation into the DNA of Rb-positive human breast (including MDA-MB-435, MCF-7), colon (H1299), and lung carcinomas (Colo-205) as well as human leukemias (CRRF-CEM and K562). In the G1 period, PD 0332991 significantly raises the percentage of MDA-MB-453.[1] In cycling CD138+ primary bone marrow myeloma cells, nontransformed primary B cells, MM1.S, and CAG HMCLs cell line, PD 0332991 inhibits phosphorylation of Rb with an IC50 of <0.1 μM, 0.05 μM, and 60-70 nM, respectively. Moreover, CD138+ primary bone marrow myeloma and nontransformed primary B cells undergo G1 arrest when treated with PD 0332991. With a ~0.05 μM IC50, PD 0332991 causes G1 arrest in MM1.S.[2] PD 0332991 specifically inhibits the growth of human breast cancer cell lines that are positive for the luminal estrogen receptor (HER2-positive included). In most sensitive lines, PD 0332991 raises the expression of the pRb and cyclin D1 genes while lowering that of CDKN2A (p16). In cell lines with conditioned resistance to ER blockade, PD 0332991 increases sensitivity to tamoxifen.[3]
|
| ln Vivo |
PD 0332991 (150 mg/kg) causes a corresponding delay in tumor growth and rapid regressions in Colo-205 colon carcinoma xenografts. In MDA-MB-435 breast cancer, PD 0332991 (150 mg/kg) causes total tumor stasis and cell death. In mice with SF-295 glioblastoma xenografts, as well as in ZR-75-1 breast and PC-3 prostate tumor models, PD 0332991 (150 mg/kg) also causes a significant tumor regression (complete suppression of tumor growth). Over the course of a full 24-hour period, PD 0332991 (150 mg/kg) suppresses Rb Ser780 phosphorylation in MDA-MB-435 breast carcinoma. In Colo-205 carcinoma xenografts, PD 0332991 (150 mg/kg) down-regulates expression of four E2F-regulated genes: CDC2, CCNE2, TK1, and TOP2A.[1] Moreover, PD 0332991 quickly stops the growth of myeloma tumors.[2]
Oral administration of PD 0332991 to mice bearing the Colo-205 human colon carcinoma produces marked tumor regression. Therapeutic doses of PD 0332991 cause elimination of phospho-Rb and the proliferative marker Ki-67 in tumor tissue and down-regulation of genes under the transcriptional control of E2F. The results indicate that inhibition of Cdk4/6 alone is sufficient to cause tumor regression and a net reduction in tumor burden in some tumors [1]. |
| Enzyme Assay |
CDK assays are run in 96-well filter plates for kinetic analysis and IC50 calculations. By infecting insect cells with baculovirus, all CDK-cyclin kinase complexes are expressed and purified. A portion of pRb fused to GST (amino acids 792–928) serves as the substrate for the assays (GST•RB-Cterm). 20 mM Tris-HCl, pH 7.4, 50 mM NaCl, 1 mM dithiothreitol, 10 mM MgCl2, 25 μM ATP (for CDK4-cyclin D1, CDK6-cyclin D2, and CDK6-cyclin D3), 0.25 μCi of [γ-32P]ATP, 20 ng of enzyme, 1 μg of GST•RB-Cterm, and suitable dilutions of inhibitor are included in the overall reaction volume of 0.1 mL. After adding all the ingredients to the wells—aside from the [γ-32P]ATP—they are put on a plate mixer for two minutes. Addition of [γ-32P]ATP initiates the reaction, which is then incubated for 15 minutes at 25°C. In order to allow the substrate to precipitate, the reaction is stopped by adding 0.1 mL of 20% trichloroacetic acid and keeping the plate at 4°C for at least an hour. After five well washes with 0.2 mL of 10% trichloroacetic acid, radioactive incorporation is measured using a β plate counter.
|
| Cell Assay |
In 24-well plates, cells are seeded in duplicate, with 5,000–10,000 cells per well. PD 0332991 is added in various concentrations the day following plating. Drug-free control wells are also seeded. Following incubation, trypsinized cells are added to isotone solution, counted right away using a Coulter Z2 particle counter.
|
| Animal Protocol |
Human colon carcinoma xenografts Colo-205
150 mg/kg o.p. injection every day Oral administration of PD 0332991 to mice bearing the Colo-205 human colon carcinoma produces marked tumor regression. Therapeutic doses of PD 0332991 cause elimination of phospho-Rb and the proliferative marker Ki-67 in tumor tissue and down-regulation of genes under the transcriptional control of E2F. The results indicate that inhibition of Cdk4/6 alone is sufficient to cause tumor regression and a net reduction in tumor burden in some tumors.[1] By specific inhibition of Cdk4/6, the orally active small-molecule PD 0332991 potently induces G(1) arrest in primary bone marrow myeloma cells ex vivo and prevents tumor growth in disseminated human myeloma xenografts. PD 0332991 inhibits Cdk4/6 proportional to the cycling status of the cells independent of cellular transformation and acts in concert with the physiologic Cdk4/6 inhibitor p18(INK4c). Inhibition of Cdk4/6 by PD 0332991 is not accompanied by induction of apoptosis. However, when used in combination with a second agent, such as dexamethasone, PD 0332991 markedly enhances the killing of myeloma cells by dexamethasone. PD 0332991, therefore, represents the first promising and specific inhibitor for therapeutic targeting of Cdk4/6 in multiple myeloma and possibly other B-cell cancers.[2] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Palbociclib presents a linear pharmacokinetic profile and its peak plasma concentration was observed 6-12 hours after oral administration. The oral bioavailability is reported to be of 46% with a steady-state reached after 8 days and a median accumulation ratio of 2.4. The absorption of palbociclib is significantly reduced under fasting conditions and hence, food intake is recommended when this drug is administered. The main route of elimination of palbociclib is through feces after hepatic metabolism while renal clearance seems to play a minor role accounting only for 17.5% of the eliminated dose. The mean apparent distribution of palbociclib is 2583 L which suggests that palbociclib penetrates extensively into peripheral tissues. The mean apparent oral clearance of palbociclib is of 63.1 L/h. Metabolism / Metabolites Palbociclib is mainly hepatically transformed. the metabolism is mainly performed by the activities of the cytochrome P450 isoenzyme 3A and the sulfotransferase 2A1. The metabolism of palbociclib is represented mainly by reactions of oxidation and sulfonation followed by acylation and glucuronidation as minor reactions. After its metabolism, palbociclib forms mainly inactive glucuronide and sulfamic acid conjugates. The major circulating metabolite, accounting for 1.5% of the dose in excreta is is the glucuronide conjugate. Biological Half-Life The mean plasma elimination half-life of palbociclib is 29 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In the large clinical trials, adverse events were common and led to dose reductions in one-third of patients and discontinuation in 8%. Publications on the efficacy and safety of palbociclib rarely mentioned serum ALT elevations or hepatotoxicity. In a study of women with refractory, metastatic breast cancer, serum ALT elevations occurred in 6% [2% over 5 times ULN] receiving palbociclib and fulvestrant compared to 3% [none over 5 times ULN] on fulvestrant alone. Since its approval and more widescale use, there have been several reports of prominent ALT elevations arising after 2 or 3 cycles of palbociclib, that improved on discontinuation and recurred rapidly when restarted. Serum bilirubin and alkaline phosphatase levels were normal and symptoms were not mentioned. In addition, there have been rare reports of patients with refractory metastatic breast cancer who developed pseudocirrhosis within 2 to 3 months of starting palbociclib presenting with fatigue, jaundice and ascites with only modest elevations in serum aminotransferase and alkaline phosphatase levels. Imaging revealed a severely nodular liver, but liver histology showed desmoplastic changes in areas of necrotic metastatic tumor without cirrhosis. The liver also had vascular changes suggestive of sinusoidal obstruction syndrome, changes possibly caused by the dramatic involution of the metastatic tumor tissue combined with vascular damage. Pseudocirrhosis has been reported with other highly successful antineoplastic therapies of cancer metastatic to the liver, but the frequency is rare. Likelihood score: C (probable rare cause of clinically apparent liver injury that may represent pseudocirrhosis from nodular transformation of the liver in response to necrosis of hepatic metastases). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of palbociclib during breastfeeding. Because palbociclib is 85% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 29 hours and it might accumulate in the infant. It is also given in combination with letrozole or fulvestrant, which may increase the risk to the infant. The manufacturer recommends that breastfeeding be discontinued during palbociclib therapy and for 3 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binding of palbociclib to human plasma proteins in vitro accounts for approximately 85% of the administered dose. |
| References | |
| Additional Infomation |
Palbociclib Isethionate is the isethionate salt form of palbociclib, an orally available cyclin-dependent kinase (CDK) inhibitor with potential antineoplastic activity. Palbociclib selectively inhibits cyclin-dependent kinase 4 (CDK4) and 6 (CDK6), thereby inhibiting retinoblastoma (Rb) protein phosphorylation early in the G1 phase leading to cell cycle arrest. This suppresses DNA replication and decreases tumor cell proliferation. CDK4 and 6 are serine/threonine kinases that are upregulated in many tumor cell types and play a key role in the regulation of cell cycle progression.
Palbociclib is a member of the class of pyridopyrimidines that is 2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7-one bearing additional methyl, acetyl and cyclopentyl substituents at positions 5, 6 and 8 respectively. It is used in combination with letrozole for the treatment of metastatic breast cancer. It has a role as an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor and an antineoplastic agent. It is a pyridopyrimidine, an aminopyridine, a secondary amino compound, a member of piperidines, an aromatic ketone, a member of cyclopentanes and a tertiary amino compound. Palbociclib is a piperazine pyridopyrimidine that acts in the cell cycle machinery. It is a second generation cyclin-dependent kinase inhibitor selected from a group of pyridopyrimidine compounds due to its favorable physical and pharmaceutical properties. Palbociclib was developed by Pfizer Inc after the discovery that identified the cyclin-dependent kinases as key regulators of cell growth. It was originally FDA approved on March 2015 for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer and its indications were updated in April 2019 to include male patients based on findings from postmarketing reports and electronic health records demonstrating safety and clinical efficacy. Palbociclib is a Kinase Inhibitor. The mechanism of action of palbociclib is as a Kinase Inhibitor, and Cytochrome P450 3A Inhibitor. Palbociclib is a unique cyclin-dependent kinase inhibitor that is used in combination with aromatase inhibitors in the treatment of postmenopausal women with metastatic breast cancer. Palbociclib is associated with transient and usually mild elevations in serum aminotransferase during therapy and to an unusual form of liver injury called pseudocirrhosis caused by shrinkage of tumor metastases in the liver combined with desmoplastic changes and vascular damage, that can be severe, progressive and even fatal. Palbociclib is an orally available cyclin-dependent kinase (CDK) inhibitor with potential antineoplastic activity. Palbociclib selectively inhibits cyclin-dependent kinase 4 (CDK4) and 6 (CDK6), thereby inhibiting retinoblastoma (Rb) protein phosphorylation early in the G1 phase leading to cell cycle arrest. This suppresses DNA replication and decreases tumor cell proliferation. CDK4 and 6 are serine/threonine kinases that are upregulated in many tumor cell types and play a key role in the regulation of cell cycle progression. See also: Palbociclib Isethionate (is active moiety of). Drug Indication Palbociclib is indicated in combination with [letrozole] as initial endocrine-based therapy for the treatment of human epidermal growth factor receptor type 2 (HER2)-negative and hormone receptor(HR)-positive tumors in adult patients with advanced/metastatic breast cancer. It is as well approved in combination with [fulvestrant] in patients with disease progression with prior endocrine therapy. In the official labeling, the use of palbociclib should be accompanied with either an aromatase inhibition, no restricted to letrozole, as initial endocrine-based therapy in postmenopausal women or in man. The breast cancer starts as a group of cancer cells that grow into and destroy the nearby breast tissue. This growth can spread into other parts of the body which is called metastasis. According to the location of the cancer cells, it can be categorized in ductal carcinoma and lobular carcinoma. However, other types of breast cancer include inflammatory breast cancer, Paget disease of the breast, triple negative breast cancer non-Hodgkin lymphoma and soft tissue sarcoma. In males, breast cancer is usually treated as the cases of postmenopausal women and almost all the cases are ductal carcinoma. FDA Label Ibrance is indicated for the treatment of hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer : in combination with an aromatase inhibitor; in combination with fulvestrant in women who have received prior endocrine therapy. In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinizing hormone releasing hormone (LHRH) agonist. Treatment of Ewing sarcoma Treatment of breast malignant neoplasms Mechanism of Action Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that acts by binding to the ATP pocket with an IC50 in the range of 9-15 nmol/L. It is important to consider that it presents low to absent activity against other kinases. The CDK4/6 kinase is involved, with coregulatory partner cyclin D, in the G1-S transition. Hence, inhibition of this step prevents cell cycle progression in cells in whose this pathway is functioning. This step includes the pathways of the phosphorylation of retinoblastoma protein and the E2F family of transcription factors. |
| Molecular Formula |
C26H35N7O6S
|
|---|---|
| Molecular Weight |
573.66
|
| Exact Mass |
573.236
|
| Elemental Analysis |
C, 54.44; H, 6.15; N, 17.09; O, 16.73; S, 5.59
|
| CAS # |
827022-33-3
|
| Related CAS # |
Palbociclib;571190-30-2;Palbociclib monohydrochloride;827022-32-2;Palbociclib dihydrochloride
|
| PubChem CID |
11478676
|
| Appearance |
Light yellow to yellow solid powder
|
| LogP |
3.379
|
| Hydrogen Bond Donor Count |
4
|
| Hydrogen Bond Acceptor Count |
12
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
40
|
| Complexity |
892
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
CC(C1=C(N2C3CCCC3)N=C(NC4=NC=C(N5CCNCC5)C=C4)N=C1)=C(C(C)=O)C2=O.OCCS(O)(=O)=O
|
| InChi Key |
LYYVFHRFIJKPOV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H29N7O2.C2H6O4S/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32;3-1-2-7(4,5)6/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29);3H,1-2H2,(H,4,5,6)
|
| Chemical Name |
6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one;2-hydroxyethanesulfonic acid
|
| Synonyms |
PD0332991 isethionate salt; PD-0332991; Palbociclib Isethionate; 827022-33-3; PD0332991 Isethionate; PD 0332991 isethionate; UNII-W1NYL2IRDR; W1NYL2IRDR; Palbociclib Isethionate [USAN]; Palbociclib (isethionate); PD 0332991; Palbociclib isethionate salt; Trade name: Ibrance
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (1.74 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: Saline: 30 mg/mL (add these co-solvents sequentially from left to right, and one by one). Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7432 mL | 8.7160 mL | 17.4319 mL | |
| 5 mM | 0.3486 mL | 1.7432 mL | 3.4864 mL | |
| 10 mM | 0.1743 mL | 0.8716 mL | 1.7432 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04693468 | Recruiting | Drug: Palbociclib Isethionate Drug: Crizotinib |
Advanced Malignant Solid Neoplasm Metastatic Malignant Solid Neoplasm |
M.D. Anderson Cancer Center | December 1, 2020 | Phase 1 |
| NCT01602887 | Completed | Drug: PD-0332991 | Healthy | Pfizer | May 2012 | Phase 1 |
| NCT02041273 | Completed | Drug: palbociclib isethionate (phase 1 and 2 studies) Drug: palbociclib commercial free base capsule |
Healthy | Pfizer | January 2014 | Phase 1 |
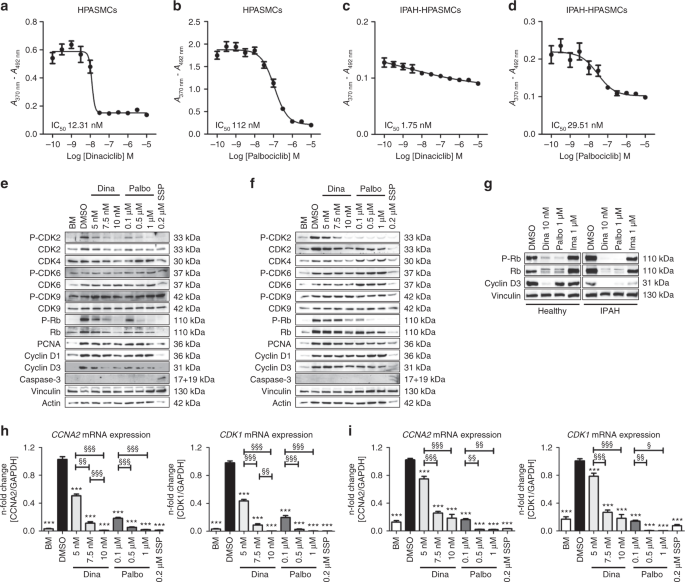 Evaluation of IC50concentrations of the CDK inhibitors dinaciclib and palbociclib on proliferation, and their effects on CDK-Rb-E2F signaling in human HPASMCs from healthy donors and IPAH patients.Nat Commun.2019May 17;10(1):2204. |
|---|
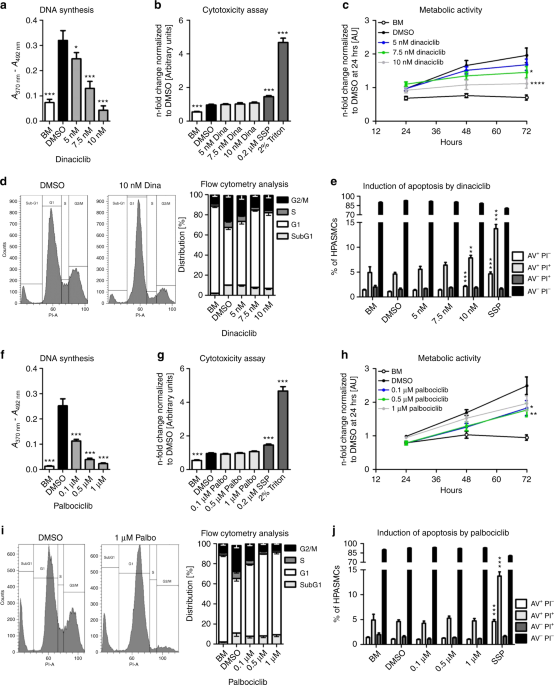 Effects of the CDK inhibitors dinaciclib and palbociclib on proliferation, cell cycle, and apoptosis.Nat Commun.2019May 17;10(1):2204. |
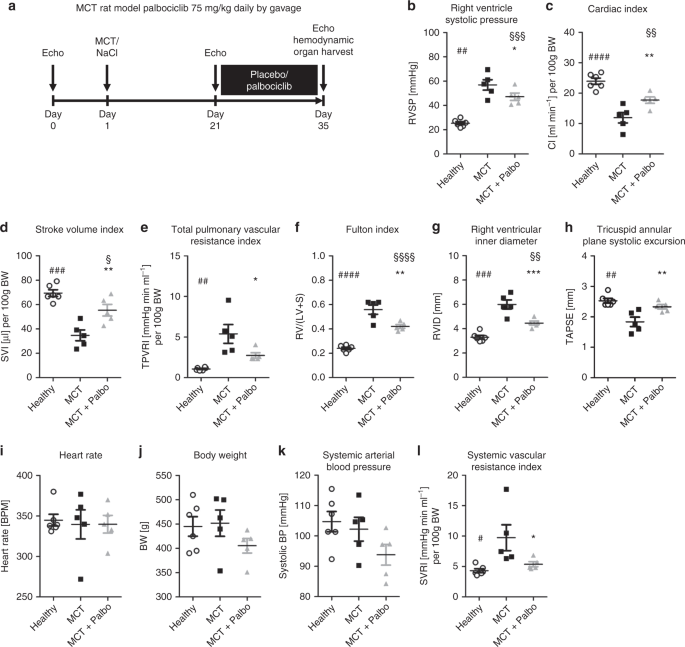 Effects of palbociclib on disease progression in the MCT rat model of pulmonary arterial hypertension.Nat Commun.2019May 17;10(1):2204. |
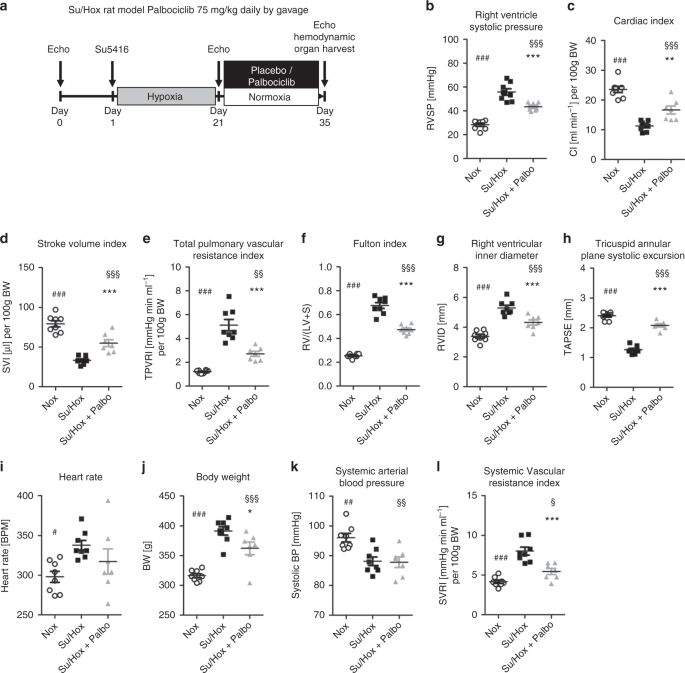 Effects of palbociclib on disease progression in the Su/Hox rat model of pulmonary arterial hypertension.Nat Commun.2019May 17;10(1):2204. |
|---|
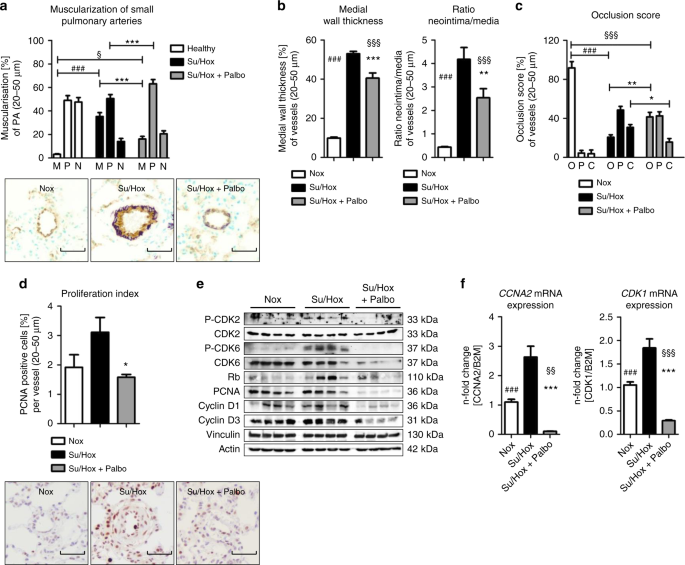 Ex vivo analyses of lung tissue for reversal of remodeling and in vivo drug efficacy in the Su/Hox model.Nat Commun.2019May 17;10(1):2204. |
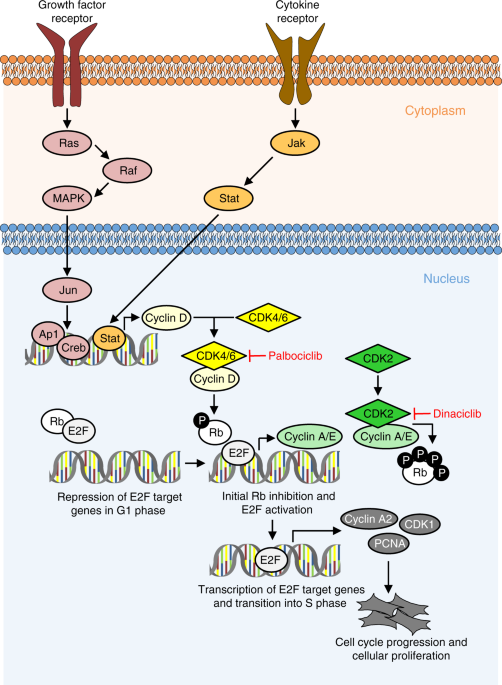 Proposed mechanism of action of palbociclib and dinaciclib in PAH. Multiple growth factors, cytokines, and mitogens induce the activation of cyclin-dependent kinases (CDKs), e.g., by increasing the expression of cyclin D1.Nat Commun.2019May 17;10(1):2204. |
|
|---|
|
|
|
|---|
|