| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| Targets |
Microtubules [1]
|
|---|---|
| ln Vitro |
A cytotoxic substance called docetaxel encourages the development of microtubule bundles, especially in cells that are proliferating. It can also cause a prolonged mitotic arrest that is followed by apoptosis or a permanent arrest of mitosis in cells that are already in a state of mitosis. Instead of stopping the cell cycle at a particular moment, docetaxel stops the dynamic instability of microtubules and the pedaling phenomenon that stops chromosomes from dividing into daughter cells. This causes the premature termination of mitosis. [2]
When the human small cell lung cancer N417 cell line is treated with docetaxel (150 ng/mL) for one hour, cell growth is 50% inhibited. IC50 values of 8 ng/mL, 4 ng/mL, 5 ng/mL, and 7 ng/mL are obtained after 96–120 hours of docetaxel treatment of KB human epidermal carcinoma cells, T24 human bladder carcinoma cells, Calc18 human breast cancer cells, and HCT116 human colorectal adenocarcinoma cells. [3] With IC50 values of 1 nM, 1 nM, 0.3 nM, 0.3 nM, 0.3 nM, 0.3 nM, 0.3 nM, 0.3 nM, respectively, docetaxel inhibited the survival of human cancer cells Hs746T (stomach), AGS (stomach), HeLa (cervix), CaSki (cervix), BxPC3 (pancreas), and Capan-1 (pancreas) following a 24-hour treatment. [4] By using the colony formation assay, docetaxel was assessed in the human ovarian cancer cell lines OVCA432, A2780, and A2780/cp8. After 12 days of treatment, the IC50 values were 0.06 nM, 0.6 nM, and 3 nM, respectively. [5] At very low concentrations, docetaxel prevents endothelial cell migration in vitro without changing the microtubules' overall shape or stopping cell division, but it has more subtle effects on microtubule dynamics. The IC50 for docetaxel is 1 pM, which inhibits HUVEC migration. With an IC 50 of 10 pM, docetaxel prevents HUVEC from chemotactically responding to vascular endothelial growth factor or the angiogenic factor thymidine phosphorylase. [7] Human monocytes were induced using docetaxel (30 μM) by incubating them for 12 hours in medium that was supplemented with 10% human serum. [8] |
| ln Vivo |
Docetaxel (33 mg/kg/dose, IV, three times every four days) in M2OL2 colon xenografts delayed tumor growth by 19.3%. Docetaxel also has potent antitumor activity against MX-1, SK-MEL-2, LX-1 and OVCAR-3 xenografts. Docetaxel inhibits the vascular response to fibroblast growth factor 2 with an IC50 of 5.4 mg/kg, and complete vascular occlusion was achieved when mice were treated with 10 mg/kg twice weekly for 14 days. Docetaxel appears to be selective for endothelial cell migration and/or microvascularization, as inflammatory cell infiltration into the basement membrane is less sensitive to inhibition by Docetaxel. [7]
|
| References |
[1]. Buey RM. Chem Biol, 2004, 11(2), 225-236. [2]. Comparative Study Biochem Biophys Res Commun. 1992 Aug 31;187(1):164-70. [3]. Chemotherapy . 2006;52(5):231-40. doi: 10.1159/000094869. Epub 2006 Aug 4. [4]. Silvestrini R, et al. Stem Cells, 1993, 11(6), 528-35. [5]. Tanaka M, et al. Eur J Cancer. 1996, 32A(2), 226-330. [6]. Mol Cancer Ther . 2002 Nov;1(13):1191-200. |
| Molecular Formula |
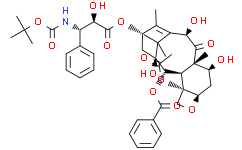
C49H55CL6NO18
|
|---|---|
| Molecular Weight |
1158.7
|
| CAS # |
114915-14-9
|
| SMILES |
CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)OC(=O)OCC(Cl)(Cl)Cl)C)OC(=O)OCC(Cl)(Cl)Cl
|
| InChi Key |
QTCVMFMWHTVJTQ-CCONUVRMSA-N
|
| InChi Code |
InChI=1S/C49H55Cl6NO18/c1-24-28(69-39(61)33(58)32(26-15-11-9-12-16-26)56-40(62)74-43(3,4)5)20-47(65)37(72-38(60)27-17-13-10-14-18-27)35-45(8,36(59)34(31(24)44(47,6)7)71-42(64)68-23-49(53,54)55)29(70-41(63)67-22-48(50,51)52)19-30-46(35,21-66-30)73-25(2)57/h9-18,28-30,32-35,37,58,65H,19-23H2,1-8H3,(H,56,62)/t28-,29-,30+,32-,33+,34+,35+,37-,45+,46-,47+/m0/s1
|
| Chemical Name |
[(1S,2S,3S,4S,7R,9S,10S,12R,15S)-4-acetyloxy-1-hydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-10,14,17,17-tetramethyl-11-oxo-9,12-bis(2,2,2-trichloroethoxycarbonyloxy)-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate
|
| Synonyms |
NSC 628503; RP56976; 7,10-o-ditroc docetaxel; 114915-14-9; [(1S,2S,3S,4S,7R,9S,10S,12R,15S)-4-Acetyloxy-1-hydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-10,14,17,17-tetramethyl-11-oxo-9,12-bis(2,2,2-trichloroethoxycarbonyloxy)-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate; 7,10-Di(trichloroethoxyformyl) docetaxel; 7,10-O-Ditrocdocetaxel; 7,11-Methano-1H-cyclodeca[3,4]benz[1,2-b]oxete, benzenepropanoic acid deriv.; 7,10-Bis(2,2,2-trichloroethoxycarbonyl)docetaxel; AKOS027327928; Benzenepropanoic acid,b-[[(1,1-dimethylethoxy)carbonyl]amino]-a-hydroxy-,12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4,6-bis[[(2,2,2-trichloroethoxy)carbonyl]oxy]-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester,[2aR-[2aa,4b,4ab,6b,9a(aR*,bS*),11a,1;
|
| Solubility (In Vitro) |
DMSO : ~100 mg/mL (~123.78 mM)
Ethanol : ~100 mg/mL (~123.78 mM) Water : Insoluble |
|---|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8630 mL | 4.3152 mL | 8.6304 mL | |
| 5 mM | 0.1726 mL | 0.8630 mL | 1.7261 mL | |
| 10 mM | 0.0863 mL | 0.4315 mL | 0.8630 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.