
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Bemcentinib (formerly known as BGB324; R428) is a novel, potent and selective inhibitor of the RTK (receptor tyrosine kinase) Axl with potential anticancer activity. With an IC50 of 14 nM, it inhibits Axl and exhibits >100-fold greater selectivity for Axl than Abl. Rat models of metastatic breast cancer demonstrated high activity of R428 in preventing tumor spread and extending survival. R428's selective Axl blockade has therapeutic value in extending the survival of animals with metastatic tumors because Axl signaling controls breast cancer metastasis at multiple levels in tumor cells and tumor stromal cells.
| Targets |
Axl kinase (IC50 = 14 nM)
|
|---|---|
| ln Vitro |
Bemcentinib (R428) (2μM) is comparable to Axl knockdown in its ability to significantly inhibit the migration and invasion mechanisms of Axlpos melanoma cells[1]. In order to improve the inhibition of liver micrometastasis, bemcentinib (R428) works in concert with CDDP#2. As demonstrated by decreased lipid uptake, bemcentinib (R428) (50 nM-1μM) inhibits preadipocyte differentiation into mature adipocytes in a concentration-dependent manner[3].
|
| ln Vivo |
After being cultured in serum-free medium for 24 hours, cells are taken out and added to the upper chamber (1.5 x 10^5 cells per well) of 24-well chambers that are either uncoated (for migration) or coated with matrigel (for invasion). The lower chamber is filled with 10% fetal bovine serum RPMI medium. Before the cells are loaded into the upper chambers, they are treated for two hours with either the vehicle (DMSO, 0.25%) or bemcentinib (R428) (2 μM). The medication or vehicle is present in both the upper and lower chambers. Using a 480/520 nm filter set on an Infinite M1000 microplate reader, the fluorescent signals of migrating or invading cells are measured 20 or 42 hours later, respectively.
|
| Enzyme Assay |
In vitro Kinase Assays[1]
A five-point R428 dose titration was performed in radiometric in vitro kinase assays on 133 kinases at the KmATP for each kinase. Axl, Mer, and Tyro3 assays were also performed using a fluorescence polarization protocol. HER2 activity was determined by Z'-LYTE assay. Axl Cell-Based Assay[1] HeLa cells were seeded in starvation medium in 96-well plates. Twenty-four hours later, cells were preincubated for 1 h with diluted R428 before stimulation with preclustered anti-Axl antibody. Cells were fixed, blocked, and stained with anti–phospho-Akt (Ser473) followed by goat anti-rabbit horseradish peroxidase before developing using SuperSignal ELISA Pico chemiluminescent substrate. See also Supplementary Materials and Methods. R428 (also known as BGB324) is a powerful and selective Axl inhibitor that is >100-fold more selective for Axl than Abl, with an IC50 of 14 nM. Additionally, R428 has a higher selectivity for Axl than Mer and Tyro3 (50–100 fold more selective) as well as InsR, EGFR, HER2, and PDGFRβ (100–fold more selective). In radiometric in vitro kinase assays, a five-point R428 dose titration was carried out on 133 kinases at the KmATP for each kinase. A fluorescence polarization protocol was also used for the Axl, Mer, and Tyro3 assays. With the Z-LYTE assay, HER2 activity was ascertained. |
| Cell Assay |
Invasion Assays[2]
MDA-MB-231 or 4T1 cells (1 × 105) were allowed to migrate through Matrigel toward 20% FCS in an 8-μm pore 24-well Transwell plate at 37°C for 16 to 24 h. Noninvaded cells and Matrigel were removed by swabbing. Invaded cells were fixed in 4% formaldehyde, stained with 1% crystal violet, and quantified as for Axl cell-based assay. Cells were preincubated with R428 for 3 h. R428 was added to both upper and lower Transwell chambers. MDA-MB-231-luc-D3H2LN Intracardiac Model[2] Seven- to 8-wk-old female NCr nu/nu mice (Taconic) were injected intracardially with bioluminescent MDA-MB-231-luc-D3H2LN cell suspension. Oral dosing with R428 (125 mg/kg) or vehicle twice daily began 2 h before cell implantation and continued to day 21 (n = 20). Metastatic burden was quantified by in vivo bioluminescence imaging on day 22 and analyzed using the Wilcoxon rank sum test. After being cultured in serum-free medium for 24 hours, cells are taken out and added to the upper chamber (1.5 x 10^5 cells per well) of 24-well chambers that are either uncoated (for migration) or coated with matrigel (for invasion). The lower chamber is filled with 10% fetal bovine serum RPMI medium. Before the cells are loaded into the upper chambers, they are treated for two hours with either the vehicle (DMSO, 0.25%) or bemcentinib (R428) (2 μM). The medication or vehicle is present in both the upper and lower chambers. Using a 480/520 nm filter set on an Infinite M1000 microplate reader, the fluorescent signals of migrating or invading cells are measured 20 or 42 hours later, respectively. |
| Animal Protocol |
Female NCr nu/nu mice aged seven to eight weeks are given intracardial injections of bioluminescent MDA-MB-231-luc-D3H2LN cell suspension. Two hours prior to cell implantation, oral dosage of Bemcentinib (R428) (125 mg/kg, p.o.) or vehicle is administered twice daily until day 21 (n=20). Day 22 in vivo bioluminescence imaging is used to measure the metastatic burden, and the Wilcoxon rank sum test is used for analysis.
Orthotopic Model[2] Female BALB/c mice were inoculated in the mammary fat pad with 0.5 × 106 4T1 cells. Forty-eight hours after inoculation, mice were randomized into treatment groups (n = 10). Oral dosing with R428 (7–75 mg/kg twice daily) or vehicle continued until days 19 to 21. Cisplatin (1.2 or 4 mg/kg) was administered i.v. once weekly. Body weight and tumor size were measured thrice per week. Lungs were exposed postmortem. Total number and size of surface lung macrometastases were measured (small, <2 mm; medium, ≥2 mm and <3 mm; large, ≥3 mm). Half of each primary tumor was snap frozen in liquid nitrogen. The other half, and the livers, were fixed in paraformaldehyde/lysine/periodate solution, paraffin embedded, and sectioned (5 μm thick). Two H&E-stained liver sections per animal were examined microscopically for micrometastases in three view fields. Synergism was determined using Clark's synergy calculation. |
| References |
|
| Additional Infomation |
Bemcentinib has been investigated for the treatment of Non-Small Cell Lung Cancer.
Bemcentinib is an orally available and selective inhibitor of the AXL receptor tyrosine kinase (UFO), with potential antineoplastic activity. Upon administration, bemcentinib targets and binds to the intracellular catalytic kinase domain of AXL and prevents its activity. This blocks AXL-mediated signal transduction pathways and inhibits the epithelial-mesenchymal transition (EMT), which, in turn, inhibits tumor cell proliferation and migration. In addition, bemcentinib enhances chemo-sensitivity. AXL, a member of the TAM (TYRO3, AXL and MER) family of receptor tyrosine kinases overexpressed by many tumor cell types, plays a key role in tumor cell proliferation, survival, invasion and metastasis; its expression is associated with drug resistance and poor prognosis. Axl, a member of the TAM (Tyro3, Axl, Mer) family of receptor tyrosine kinases, displays an increasingly important role in carcinogenesis. Analysis of 58 cutaneous melanoma lines indicated that Axl was expressed in 38% of them, with significant overrepresentation in NRAS- compared with BRAF-mutated tumors. Axl activation could be induced by autocrine production of its ligand, Gas6, in a significant fraction of Axl-positive tumors. Pearson's correlation analysis on expression data from five data sets of melanoma lines identified several transcripts correlating positively or negatively with Axl. By functionally grouping genes, those inversely correlated were involved in melanocyte development and pigmentation, whereas those positively correlated were involved in motility, invasion, and microenvironment interactions. Accordingly, Axl-positive melanomas did not express microphthalmia transcription factor (MITF) and melanocyte differentiation antigens (MDAs) such as MART-1 and gp100 and possessed a greater in vitro invasive potential compared with Axl-negative ones. Motility, invasivity, and ability to heal a wound or to migrate across an endothelial barrier were inhibited in vitro by Axl knockdown. Pharmacological inhibition of Axl using the selective inhibitor R428 had comparable effects in reducing migration and invasion. These results suggest that targeted inhibition of Axl signaling in the subset of melanomas lacking MITF and MDAs may represent a novel therapeutic strategy.[1] Accumulating evidence suggests important roles for the receptor tyrosine kinase Axl in cancer progression, invasion, metastasis, drug resistance, and patient mortality, highlighting Axl as an attractive target for therapeutic development. We have generated and characterized a potent and selective small-molecule inhibitor, R428, that blocks the catalytic and procancerous activities of Axl. R428 inhibits Axl with low nanomolar activity and blocked Axl-dependent events, including Akt phosphorylation, breast cancer cell invasion, and proinflammatory cytokine production. Pharmacologic investigations revealed favorable exposure after oral administration such that R428-treated tumors displayed a dose-dependent reduction in expression of the cytokine granulocyte macrophage colony-stimulating factor and the epithelial-mesenchymal transition transcriptional regulator Snail. In support of an earlier study, R428 inhibited angiogenesis in corneal micropocket and tumor models. R428 administration reduced metastatic burden and extended survival in MDA-MB-231 intracardiac and 4T1 orthotopic (median survival, >80 days compared with 52 days; P < 0.05) mouse models of breast cancer metastasis. Additionally, R428 synergized with cisplatin to enhance suppression of liver micrometastasis. Our results show that Axl signaling regulates breast cancer metastasis at multiple levels in tumor cells and tumor stromal cells and that selective Axl blockade confers therapeutic value in prolonging survival of animals bearing metastatic tumors.[2] A low-molecular-weight receptor tyrosine kinase inhibitor, 1-(6,7-dihydro-5H-benzo(6,7)cyclohepta(1,2-c)pyridazin-3-yl)-N3-((7-pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo(7)annulene-2-yl)-1H-1,2,4-triazole-3,5-diamine (R428) with high affinity and selectivity for the growth arrest-specific protein 6 (GAS6) receptor Axl was used to study a potential role of GAS6 signaling in adiposity. In vitro, R428 caused a concentration-dependent inhibition of preadipocyte differentiation into mature adipocytes, as evidenced by reduced lipid uptake. Inhibition of Axl-mediated signaling was confirmed by reduced levels of phospho-Akt activity. In vivo, oral administration of R428 for 5 weeks to mice kept on a high-fat diet resulted in significantly reduced weight gain and subcutaneous and gonadal fat mass. This was associated with marked adipocyte hypotrophy, enhanced macrophage infiltration, and apoptosis. Thus, affecting GAS6 signaling through receptor antagonism using a low-molecular-weight Axl antagonist impairs adipocyte differentiation and reduces adipose tissue development in a murine model of nutritionally induced obesity.[3] |
| Molecular Formula |
C30H34N8
|
|
|---|---|---|
| Molecular Weight |
506.64
|
|
| Exact Mass |
506.29
|
|
| Elemental Analysis |
C, 71.12; H, 6.76; N, 22.12
|
|
| CAS # |
1037624-75-1
|
|
| Related CAS # |
1037624-75-1; 1037624-91-1 (racemic);
|
|
| PubChem CID |
46215462
|
|
| Appearance |
Off-white to yellow solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Boiling Point |
799.6±70.0 °C at 760 mmHg
|
|
| Flash Point |
437.4±35.7 °C
|
|
| Vapour Pressure |
0.0±2.8 mmHg at 25°C
|
|
| Index of Refraction |
1.768
|
|
| LogP |
4.55
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
7
|
|
| Rotatable Bond Count |
4
|
|
| Heavy Atom Count |
38
|
|
| Complexity |
775
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
N1(C([H])([H])C([H])([H])C([H])([H])C1([H])[H])[C@@]1([H])C([H])([H])C([H])([H])C2C([H])=C([H])C(=C([H])C=2C([H])([H])C1([H])[H])N([H])C1N=C(N([H])[H])N(C2C([H])=C3C(C4=C([H])C([H])=C([H])C([H])=C4C([H])([H])C([H])([H])C3([H])[H])=NN=2)N=1
|
|
| InChi Key |
KXMZDGSRSGHMMK-VWLOTQADSA-N
|
|
| InChi Code |
InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1
|
|
| Chemical Name |
1-(3,4-diazatricyclo[9.4.0.02,7]pentadeca-1(15),2,4,6,11,13-hexaen-5-yl)-3-N-[(7S)-7-pyrrolidin-1-yl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-3-yl]-1,2,4-triazole-3,5-diamine
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.08 mg/mL (4.11 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: 5% DMSO+corn oil: 1 mg/mL View More
Solubility in Formulation 3: 12.5 mg/mL (24.67 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Solubility in Formulation 4: 5 mg/mL (9.87 mM) in 0.5%HPMC 1%Tween80 (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9738 mL | 9.8689 mL | 19.7379 mL | |
| 5 mM | 0.3948 mL | 1.9738 mL | 3.9476 mL | |
| 10 mM | 0.1974 mL | 0.9869 mL | 1.9738 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
 |
 |
A, experimental protocol for MDA-MB-231-luc-D3H2LN metastasis prevention study.Cancer Res.2010 Feb 15;70(4):1544-54. |
A, experimental protocol for 4T1 orthotopic metastasis study.Cancer Res.2010 Feb 15;70(4):1544-54. |
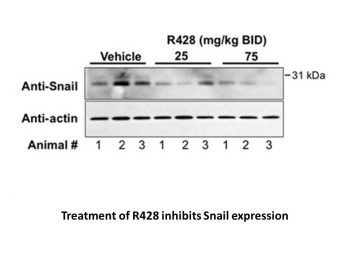
R428 modulates expression of Snail and GM-CSF in 4T1 primary tumors.Cancer Res.2010 Feb 15;70(4):1544-54. |