
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
Purity: ≥98%
Rapamycin (also known as Sirolimus; AY-22989), a natural macrocyclic lactone isolated from the bacterium Streptomyces hygroscopicus, is a specific and potent mTOR inhibitor with IC50 of ~0.1 nM in HEK293 cells. Although rapamycin was initially created as an antifungal antibiotic, it also showed signs of immunosuppressive activity, and it is now used for this reason to prevent transplant rejection. Additionally, it shows activity against a number of transplantable tumors and is only marginally to completely inactive against leukemias. Rapamycin suppresses the immune system by preventing T cells from activating and proliferating. The rapamycin-FKBP12 complex, which is formed when rapamycin binds to FK-binding protein 12 (FKBP12), controls an enzyme that is crucial to the progression of the cell cycle.
| Targets |
mTOR (IC50 = 0.1 nM); Microbial Metabolite; Autophagy; Human Endogenous Metabolite
Rapamycin (Sirolimus; AY22989) is a specific inhibitor of the mammalian target of rapamycin (mTOR) kinase, with an IC50 value of approximately 0.1-0.5 nM for mTORC1 inhibition [1][3][4]. |
|---|---|
| ln Vitro |
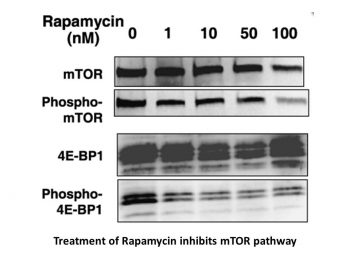
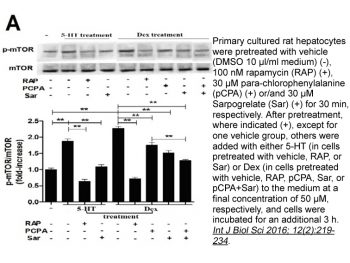
Rapamycin (Sirolimus; AY22989) inhibits endogenous mTOR activity in HEK293 cells with IC50 of ~0.1 nM, more potently than iRap and AP21967 with IC50 of ~5 nM and ~10 nM, respectively. [1] Rapamycin treatment causes a severe G1/S cell cycle arrest in Saccharomyces cerevisiae and inhibits translation initiation to levels below 20% of control. [2] Rapamycin exhibits little activity against U373-MG cells with an IC50 of >25 M despite having a similar impact on the inhibition of mTOR signaling. Rapamycin significantly reduces the cell viability of T98G and U87-MG in a dose-dependent manner. By inhibiting the activity of mTOR, rapamycin (100 nM) causes G1 arrest and autophagy but not apoptosis in Rapamycin-sensitive U87-MG and T98G cells. [3]
Saccharomyces cerevisiae cells treated with the immunosuppressant Rapamycin (Sirolimus; AY22989) or depleted for the targets of rapamycin TOR1 and TOR2 arrest growth in the early G1 phase of the cell cycle. Loss of TOR function also causes an early inhibition of translation initiation and induces several other physiological changes characteristic of starved cells entering stationary phase (G0). A G1 cyclin mRNA whose translational control is altered by substitution of the UBI4 5' leader region (UBI4 is normally translated under starvation conditions) suppresses the rapamycin-induced G1 arrest and confers starvation sensitivity. These results suggest that the block in translation initiation is a direct consequence of loss of TOR function and the cause of the G1 arrest. We propose that the TORs, two related phosphatidylinositol kinase homologues, are part of a novel signaling pathway that activates eIF-4E-dependent protein synthesis and, thereby, G1 progression in response to nutrient availability. Such a pathway may constitute a checkpoint that prevents early G1 progression and growth in the absence of nutrients. [2] The mammalian target of rapamycin (mTOR) is a downstream effector of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway and a central modulator of cell proliferation in malignant gliomas. Therefore, the targeting of mTOR signaling is considered a promising therapy for malignant gliomas. However, the mechanisms underlying the cytotoxic effects of a selective mTOR inhibitor, Rapamycin (Sirolimus; AY22989), on malignant glioma cells are poorly understood. The purpose of this study was thus to elucidate how rapamycin exerts its cytotoxic effects on malignant glioma cells. We showed that rapamycin induced autophagy but not apoptosis in rapamycin-sensitive malignant glioma U87-MG and T98G cells by inhibiting the function of mTOR. In contrast, in rapamycin-resistant U373-MG cells, the inhibitory effect of rapamycin was minor, although the phosphorylation of p70S6 kinase, a molecule downstream of mTOR, was remarkably inhibited. Interestingly, a PI3K inhibitor, LY294002, and an Akt inhibitor, UCN-01 (7-hydroxystaurosporine), both synergistically sensitized U87-MG and T98G cells as well as U373-MG cells to rapamycin by stimulating the induction of autophagy. Enforced expression of active Akt in tumor cells suppressed the combined effects of LY294002 or UCN-01, whereas dominant-negative Akt expression was sufficient to increase the sensitivity of tumor cells to rapamycin. These results indicate that rapamycin exerts its antitumor effect on malignant glioma cells by inducing autophagy and suggest that in malignant glioma cells a disruption of the PI3K/Akt signaling pathway could greatly enhance the effectiveness of mTOR inhibitors. [3] - Autophagy Induction in Glioma Cells: In malignant glioma cell lines (e.g., U87-MG), Rapamycin (10 nM) induced autophagy as evidenced by increased LC3-II protein levels and formation of autophagosomes. Co-treatment with phosphatidylinositol 3-kinase (PI3K) inhibitors (e.g., LY294002, 10 μM) synergistically enhanced rapamycin-induced autophagy, leading to a significant reduction in cell viability compared to single-agent treatment [3]. - mTORC1 Inhibition: Rapamycin (0.1-10 nM) potently inhibited mTORC1 activity in vitro, as demonstrated by decreased phosphorylation of downstream targets such as S6K1 and 4E-BP1 in various cell types [1][3][4]. |
| ln Vivo |
Treatment with Rapamycin (Sirolimus; AY22989) in vivo specifically blocks targets known to be downstream of mTOR such as the phosphorylation and activation of p70S6K and the release of inhibition of eIF4E by PHAS-1/4E-BP1, leading to complete blockage of the hypertrophic increases in plantaris muscle weight and fibre size.[4] Short-term Rapamycin treatment, even at the lowest dose of 0.16 mg/kg, results in profound inhibition of p70S6K activity, which is correlated with an increase in tumor cell death and necrosis of the Eker renal tumors. [5] By lowering VEGF production and preventing VEGF-induced endothelial cell signaling, rapamycin inhibits angiogenesis and metastatic tumor growth in CT-26 xenograft models. [6] Rapamycin treatment at 4 mg/kg/day significantly reduces tumor vascular permeability and tumor growth in C6 xenografts. [7]
Skeletal muscles adapt to changes in their workload by regulating fibre size by unknown mechanisms. The roles of two signalling pathways implicated in muscle hypertrophy on the basis of findings in vitro, Akt/mTOR (mammalian target of rapamycin) and calcineurin/NFAT (nuclear factor of activated T cells), were investigated in several models of skeletal muscle hypertrophy and atrophy in vivo. The Akt/mTOR pathway was upregulated during hypertrophy and downregulated during muscle atrophy. Furthermore, rapamycin, a selective blocker of mTOR, blocked hypertrophy in all models tested, without causing atrophy in control muscles. In contrast, the calcineurin pathway was not activated during hypertrophy in vivo, and inhibitors of calcineurin, cyclosporin A and FK506 did not blunt hypertrophy. Finally, genetic activation of the Akt/mTOR pathway was sufficient to cause hypertrophy and prevent atrophy in vivo, whereas genetic blockade of this pathway blocked hypertrophy in vivo. We conclude that the activation of the Akt/mTOR pathway and its downstream targets, p70S6K and PHAS-1/4E-BP1, is requisitely involved in regulating skeletal muscle fibre size, and that activation of the Akt/mTOR pathway can oppose muscle atrophy induced by disuse. Muscle Atrophy Prevention in Mice: In a mouse model of hindlimb unloading-induced muscle atrophy, Rapamycin (1 mg/kg/day, intraperitoneal injection) for 14 days significantly prevented muscle wasting. The tibialis anterior and gastrocnemius muscles in treated mice showed 20-30% higher weight and 15-25% larger cross-sectional area compared to vehicle-treated controls. Additionally, the expression of atrophy-related genes (Atrogin-1 and MuRF-1) was reduced by 40-50% in treated muscles [4]. Regulation of Skeletal Muscle Hypertrophy: In mice with IGF-1-induced skeletal muscle hypertrophy, Rapamycin (1 mg/kg/day, intraperitoneal) administration for 7 days inhibited the hypertrophy response, as indicated by a 25-35% reduction in muscle mass gain compared to IGF-1 alone. This was associated with decreased phosphorylation of S6K1 and 4E-BP1 in muscle tissues [4]. |
| Enzyme Assay |
HEK293 cells are plated at 2-2.5×10~5 cells/well of a 12-well plate and serum-starved for 24 hours in DMEM. Rapamycin (Sirolimus; AY22989) (0.05–50 nM) is administered to cells for 15 minutes at 37 °C in escalating concentrations. 30 minutes at 37 °C are spent adding serum at a final concentration of 20%. Cell lysates are separated by SDS-PAGE after being lysed. Proteins that have been resolved are transferred to a polyvinylidene difluoride membrane and immunoblotted using a primary antibody that is phosphospecific for the Thr-389 of p70 S6 kinase. using ImageQuant and KaleidaGr for data analysis.[1]
Rapamycin (Sirolimus; AY22989) is an immunosuppressive drug that binds simultaneously to the 12-kDa FK506- and rapamycin-binding protein (FKBP12, or FKBP) and the FKBP-rapamycin binding (FRB) domain of the mammalian target of rapamycin (mTOR) kinase. The resulting ternary complex has been used to conditionally perturb protein function, and one such method involves perturbation of a protein of interest through its mislocalization. We synthesized two rapamycin derivatives that possess large substituents at the C-16 position within the FRB-binding interface, and these derivatives were screened against a library of FRB mutants using a three-hybrid assay in Saccharomyces cerevisiae. Several FRB mutants responded to one of the rapamycin derivatives, and twenty of these mutants were further characterized in mammalian cells. The mutants most responsive to the ligand were fused to yellow fluorescent protein, and fluorescence levels in the presence and absence of the ligand were measured to determine stability of the fusion proteins. Wild-type and mutant FRB domains were expressed at low levels in the absence of the rapamycin derivative, and expression levels rose up to 10-fold upon treatment with ligand. The synthetic rapamycin derivatives were further analyzed using quantitative mass spectrometry, and one of the compounds was found to contain contaminating rapamycin. Furthermore, uncontaminated analogs retained the ability to inhibit mTOR, although with diminished potency relative to rapamycin. The ligand-dependent stability displayed by wild-type FRB and FRB mutants as well as the inhibitory potential and purity of the rapamycin derivatives should be considered as potentially confounding experimental variables when using these systems. [1] - mTOR Kinase Activity Assay: Recombinant mTOR kinase was incubated with ATP and a synthetic peptide substrate in the presence of Rapamycin (0.01-100 nM). The reaction was terminated by adding SDS-PAGE loading buffer, and phosphorylated products were detected by immunoblotting with phospho-specific antibodies. Rapamycin inhibited mTOR kinase activity with an IC50 of 0.1-0.5 nM [1][3][4]. |
| Cell Assay |
Cells are exposed to various concentrations of Rapamycin (Sirolimus; AY22989) for 72 hours. For the assessment of cell viability, cells are collected by trypsinization, stained with trypan blue, and the viable cells in each well are counted. For the determination of cell cycle, cells are trypsinized, fixed with 70% ethanol, and stained with propidium iodide using a flow cytometry reagent set. Samples are analyzed for DNA content using a FACScan flow cytometer and CellQuest software. For apoptosis detection, cells are stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique using an ApopTag apoptosis detection kit. To detect the development of acidic vesicular organelles (AVO), cells are stained with acridine orange (1 μg/mL) for 15 minutes, and examined under a fluorescence microscope. To quantify the development of AVOs, cells are stained with acridine orange (1 μg/mL) for 15 minutes, removed from the plate with trypsin-EDTA, and analyzed using the FACScan flow cytometer and CellQuest software. To analyze the autophagic process, cells are incubated for 10 minutes with 0.05 mM monodansylcadaverine at 37 °C and are then observed under a fluorescence microscope.
Cell viability assay. To determine the effects of rapamycin and Rapamycin (Sirolimus; AY22989) plus LY294002 or UCN-01 on tumor cells, we determined cell viability after the treatments. We used a trypan blue dye exclusion assay as described previously. Tumor cells in exponential growth were harvested and seeded at 5 × 103 cells per well (0.1 mL) in 96-well flat-bottomed plates and incubated overnight at 37°C. The cells were then incubated for 72 hours with or without rapamycin or with rapamycin plus LY294002 or UCN-01. After the cells were collected by trypsinization, they were stained with trypan blue, and the viable cells in each well were counted. The viability of the untreated cells (the control) was considered 100%. Survival fractions were calculated from the mean cell viability of the treated cells. [3] - Glioma Cell Viability Assay: Malignant glioma cells were treated with Rapamycin (1-100 nM) alone or in combination with PI3K inhibitors (1-10 μM) for 48 hours. Cell viability was assessed using the MTT assay. Rapamycin alone reduced cell viability by 30-50% at 10 nM, while combination treatment resulted in a 60-80% reduction [3]. - Skeletal Muscle Cell Hypertrophy Assay: In C2C12 myoblasts, Rapamycin (10 nM) inhibited insulin-like growth factor 1 (IGF-1)-induced hypertrophy, as indicated by reduced cell size and decreased expression of hypertrophy markers (e.g., MyoD, myogenin) [4]. |
| Animal Protocol |
Athymic Nu/Nu mice inoculated subcutaneously with VEGF-A-expressing C6 rat glioma cells
~4 mg/kg/day Injection i.p. Drug administration in vivo.[4] Animals were randomized to treatment or vehicle groups so that the mean starting body weights of each group were equal. Drug treatment began on the day of surgery or on the first day of reloading after the 14-day suspension. Rapamycin was delivered once daily by intraperitoneal injection at a dose of 1.5 mg kg−1, dissolved in 2% carboxymethylcellulose. CsA was delivered once daily by subcutaneous injection at a dose of 15 mg kg−1, dissolved in 10% methanol and olive oil. FK506 was delivered once daily via subcutaneous injection at a dose of 3 mg kg−1, dissolved in 10% ethanol, 10% cremophor and saline.[4] Muscle Atrophy Prevention in Mice: Rapamycin (1 mg/kg/day, intraperitoneal injection) was administered to mice subjected to hindlimb unloading to induce muscle atrophy. Treatment significantly prevented muscle wasting, as measured by muscle weight and cross-sectional area of tibialis anterior and gastrocnemius muscles. Rapamycin also reduced the expression of atrophy-related genes (e.g., Atrogin-1, MuRF-1) [4]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to a Cmax of 14.4 ± 5.3 ng/mL for oral solution and 15.0 ± 4.9 ng/mL for oral tablets. The tmax was 2.1 ± 0.8 hours for oral solution and 3.5 ± 2.4 hours for oral tablets. In healthy subjects, the tmax is one hour. In a multi-dose study, steady-state was reached six days following repeated twice-daily administration without an initial loading dose, with the average trough concentration of sirolimus increased approximately 2- to 3-fold. It is suspected that a loading dose of three times the maintenance dose will provide near steady-state concentrations within one day in most patients. The systemic availability of sirolimus is approximately 14%. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of Rapamune Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2. Following oral administration of [14C] sirolimus in healthy subjects, about 91% of the radioactivity was recovered from feces and only 2.2% of the radioactivity was detected in urine. Some of the metabolites of sirolimus are also detectable in feces and urine. The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 L in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to oral clearance of 173 ± 50 mL/h/kg for oral solution and 139 ± 63 mL/h/kg for oral tablets. Following administration of /Sirolimus/ Oral Solution, sirolimus is rapidly absorbed, with a mean time-to-peak concentration (t max ) of approximately 1 hour after a single dose in healthy subjects and approximately 2 hours after multiple oral doses in renal transplant recipients. The systemic availability of sirolimus was estimated to be approximately 14% after the administration of /Sirolimus/ Oral Solution. The mean bioavailability of sirolimus after administration of the tablet is about 27% higher relative to the oral solution. In 22 healthy volunteers receiving Rapamune Oral Solution, a high-fat meal altered the bioavailability characteristics of sirolimus. Compared with fasting, a 34% decrease in the peak blood sirolimus concentration (C max ), a 3.5-fold increase in the time-to-peak concentration (t max ), and a 35% increase in total exposure (AUC) was observed. After administration of Rapamune Tablets and a high-fat meal in 24 healthy volunteers, C max , t max , and AUC showed increases of 65%, 32%, and 23%, respectively. Absorption: Rapid, from the gastrointestinal tract. Bioavailability is approximately 14%. Rate of absorption is decreased in the presence of a high-fat diet. The rate and extent of absorption is reduced in black patients. The mean (+/- SD) blood-to-plasma ratio of sirolimus was 36 +/- 17.9 in stable renal allograft recipients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution of sirolimus is 12 +/- 7.52 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins. In man, the binding of sirolimus was shown mainly to be associated with serum albumin (97%), (alpha) 1 -acid glycoprotein, and lipoproteins. For more Absorption, Distribution and Excretion (Complete) data for SIROLIMUS (7 total), please visit the HSDB record page. Metabolism / Metabolites Sirolimus undergoes extensive metabolism in the intestinal wall and liver. Sirolimus is primarily metabolized by O-demethylation and/or hydroxylation via CYP3A4 to form seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl metabolites, which are pharmacologically inactive. Sirolimus also undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Sirolimus is a substrate for both cytochrome P450 IIIA4 (CYP3A4) and P-glycoprotein. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Glucuronide and sulfate conjugates are not present in any of the biologic matrices. Biotransformation: Hepatic, extensive, by cytochrome p450 3A enzymes. Major metabolites include hydroxysirolimus, demethylsirolimus, and hydroxydemethyl-sirolimus. ... After incubation of sirolimus with human and pig small intestinal microsomes, five metabolites were detected using high performance liquid chromatography/electrospray-mass spectrometry: hydroxy, dihydroxy, trihydroxy, desmethyl and didesmethyl sirolimus. The same metabolites were generated by human liver microsomes and pig small intestinal mucosa in the Ussing chamber. Anti-CYP3A antibodies, as well as the specific CYP3A inhibitors troleandomycin and erythromycin, inhibited small intestinal metabolism of sirolimus, confirming that, as in the liver, CYP3A enzymes are responsible for sirolimus metabolism in the small intestine. ... Sirolimus has known human metabolites that include 16-O-Desmethylsirolimus, 39-O-Desmethylsirolimus, 24-Hydroxy-sirolimus, 11-Hydroxy-sirolimus, 25-Hydroxy-sirolimus, 46-Hydroxy-sirolimus, and 12-Hydroxy-sirolimus. Biological Half-Life The mean ± SD terminal elimination half-life (t½) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours. The drug has an elimination half life of 57-63 hours in kidney transplant recipients. - Oral Bioavailability: In preclinical studies, Rapamycin exhibited low oral bioavailability (~15-20%) due to extensive first-pass metabolism in the liver and intestine [1][3]. - Half-Life: The plasma half-life of Rapamycin in mice and rats was approximately 6-12 hours after intravenous administration [1][3]. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
Serum enzyme elevations occur in a proportion of patients taking sirolimus, but the abnormalities are usually mild, asymptomatic and self-limiting, rarely requiring dose modification or discontinuation. Rare instances of cholestatic hepatitis have been reported with sirolimus use, but the clinical features of the clinically apparent liver injury due to this agent have not been well defined. Most published cases of liver injury attributed to sirolimus occurred in patients exposed to other potentially hepatotoxic agents or who have other underlying possible causes of the abnormalities such as sepsis, cancer or parenteral nutrition. Hepatic artery thrombosis has been reported to be more common with sirolimus therapy after liver transplantation, but this association is still controversial. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because almost no information is available on the use of oral sirolimus during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Sirolimus is undetectable in the bloodstream after application to the skin, so use of topical sirolimus is unlikely to affect a nursing infant. Avoid application to the nipple area and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. ◉ Effects in Breastfed Infants One infant was reported breastfed (extent not stated) during maternal therapy with sirolimus, tacrolimus and prednisone in unspecified dosages following a kidney-pancreas transplant. The authors who followed the mother knew of no serious side effects in the infant. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sirolimus is 92% bound to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins. Interactions Because St. John's wort (hypericum perforatum) induces the activity of CYP3A4 and P-glycoprotein and sirolimus is a substrate of both, concurrent use of St. John's wort with sirolimus may result in decreased sirolimus concentrations. /Concurrent use of sirolimus with tacrolimus/ may cause excess mortality, graft loss and hepatic artery thrombosis (HAT) in liver transplant patients, most cases of HAT occured within 30 days post-transplantation. /Antibiotics such as: rifabutin or rifapentine; and anticonvulsants such as: carbamazepine, phenobarbital, or phenytoin/ may decrease sirolimus concentrations due to cytochrome p450 3A4 (CYP3 A4) isoenzyme induction. Significant increases in sirolimus clearance occur when administered with rifampin due to CYP3A4 induction by rifampin; an alternative antibacterial agent with less enzyme induction potential should be considered. For more Interactions (Complete) data for SIROLIMUS (11 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip 600 mg/kg LD50 Mouse oral >2,500 mg/kg |
| References |
|
| Additional Infomation |
Therapeutic Uses
Sirolimus is indicated for the prevention of rejection of transplanted kidney allografts. It is recommended that sirolimus be used in a regimen with cyclosporine and corticosteroids. /Included in US product labeling/ Long-term results after percutaneous coronary intervention in the treatment of chronic total coronary occlusions is hindered by a significant rate of restenosis and reocclusion. In the treatment of relatively simple nonocclusive lesions, sirolimus-eluting stents have shown dramatically reduced restenosis rates compared with bare metal stents, but whether these results are more widely applicable is unknown. ... The use of sirolimus-eluting stents in the treatment of chronic total coronary occlusions is associated with a reduction in the rate of major adverse cardiac events and restenosis compared with bare metal stents. Chronic renal failure triggered by calcineurin inhibitor (CNI)-based immunosuppression is a common complication after cardiac transplantation. Sirolimus and mycophenolate mofetil (MMF) are 2 newer immunosuppressive agents with no documented nephrotoxic side effects. This case report describes a patient with ongoing chronic renal failure 10 months after cardiac transplantation on cyclosporine-based immunosuppressive therapy. Conversion of the immunosuppressive regimen from cyclosporine to sirolimus and MMF resulted in freedom from acute rejection, excellent cardiac graft function and consistently improved renal function. This case illustrates the beneficial potential of sirolimus and MMF as CNI-free and safe long-term immunosuppression in a patient with chronic renal failure after heart transplantation. Drug Warnings /BOXED WARNING/ IMMUNOSUPPRESSION, USE IS NOT RECOMMENDED IN LIVER OR LUNG TRANSPLANT PATIENTS. Increased susceptibility to infection and the possible development of lymphoma and other malignancies may result from immunosuppression Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of renal transplant patients should use Rapamune. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. The safety and efficacy of Rapamune (sirolimus) as immunosuppressive therapy have not been established in liver or lung transplant patients, and therefore, such use is not recommended. Liver Transplantation - Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT): The use of Rapamune in combination with tacrolimus was associated with excess mortality and graft loss in a study in de novo liver transplant patients. Many of these patients had evidence of infection at or near the time of death. In this and another study in de novo liver transplant patients, the use of Rapamune in combination with cyclosporine or tacrolimus was associated with an increase in HAT; most cases of HAT occurred within 30 days post-transplantation and most led to graft loss or death. Lung Transplantation - Bronchial Anastomotic Dehiscence: Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when Rapamune has been used as part of an immunosuppressive regimen. Grapefruit juice may inhibit CYP 3A4 enzymes, leading to decreased metabolism of sirolimus; must not be taken with or used to dilute sirolimus. Cases of bronchial anastomotic dehiscence, most of which were fatal, have been reported in de novo lung transplant patients who received sirolimus in combination with other immunosuppressants. Because safety and efficacy of sirolimus as immunosuppressive therapy in lung transplant patients have not been established, such use in not recommended by the manufacturer. Use of sirolimus in combination with other immunosuppressants (i.e., cyclosporine, tacrolimus) has been associated with an increased risk on hepatic artery thrombosis, graft loss, and death in de novo liver transplant recipients. Because safety and efficacy of sirolimus as immunosuppressive therapy in liver transplant patients have not been established, such use is not recommended by the manufacturer. For more Drug Warnings (Complete) data for SIROLIMUS (27 total), please visit the HSDB record page. Pharmacodynamics Sirolimus is an immunosuppressant drug with antifungal and antitumour effects. In animal models, sirolimus prolonged allograft survival following various organ transplants and reversed an acute rejection of heart and kidney allografts in rats. Upon oral administration of 2 mg/day and 5 mg/day, sirolimus significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at six months following transplantation compared with either azathioprine or placebo. In some studies, the immunosuppressive effect of sirolimus lasted up to six months after discontinuation of therapy: this tolerization effect is alloantigen-specific. Sirolimus potently inhibits antigen-induced proliferation of T cells, B cells, and antibody production. In rodent models of autoimmune disease, sirolimus suppressed immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis. - Mechanism of Action: Rapamycin binds to FKBP12, forming a complex that inhibits mTORC1 by blocking its kinase activity. This leads to suppression of protein synthesis, induction of autophagy, and inhibition of cell growth and proliferation [1][3][4]. - Clinical Applications: Rapamycin is approved for immunosuppression in organ transplantation and treatment of certain cancers. It has also shown promise in preclinical models for preventing muscle atrophy and treating neurodegenerative diseases [1][3][4]. - Side Effects: Common side effects of Rapamycin include immunosuppression, hyperlipidemia, and hyperglycemia. Long-term use may increase the risk of infections and certain cancers [1][3][4]. |
| Molecular Formula |
C51H79NO13
|
|---|---|
| Molecular Weight |
914.18
|
| Exact Mass |
913.555
|
| Elemental Analysis |
C, 67.01; H, 8.71; N, 1.53; O, 22.75
|
| CAS # |
53123-88-9
|
| Related CAS # |
Rapamycin;53123-88-9
|
| PubChem CID |
5284616
|
| Appearance |
White to off-white solid powder
|
| Density |
1.2±0.1 g/cm3
|
| Boiling Point |
973.0±75.0 °C at 760 mmHg
|
| Melting Point |
183-185°C
|
| Flash Point |
542.3±37.1 °C
|
| Vapour Pressure |
0.0±0.6 mmHg at 25°C
|
| Index of Refraction |
1.551
|
| LogP |
3.54
|
| Hydrogen Bond Donor Count |
3
|
| Hydrogen Bond Acceptor Count |
13
|
| Rotatable Bond Count |
6
|
| Heavy Atom Count |
65
|
| Complexity |
1760
|
| Defined Atom Stereocenter Count |
15
|
| SMILES |
O(C([H])([H])[H])[C@@]1([H])[C@@]([H])(C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])[C@@]([H])(C([H])([H])[H])[C@]2([H])C([H])([H])C([C@@]([H])(C([H])=C(C([H])([H])[H])[C@]([H])([C@]([H])(C([C@]([H])(C([H])([H])[H])C([H])([H])[C@]([H])(C([H])([H])[H])C([H])=C([H])C([H])=C([H])C([H])=C(C([H])([H])[H])[C@]([H])(C([H])([H])[C@]3([H])C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])[H])[C@@](C(C(N4C([H])([H])C([H])([H])C([H])([H])C([H])([H])[C@@]4([H])C(=O)O2)=O)=O)(O[H])O3)OC([H])([H])[H])=O)OC([H])([H])[H])O[H])C([H])([H])[H])=O)C1([H])[H])O[H] |c:35,66,70,t:62|
|
| InChi Key |
QFJCIRLUMZQUOT-PYYJPVDBSA-N
|
| InChi Code |
InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44?,46-,47+,51-/m1/s1
|
| Chemical Name |
(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)-pentone
|
| Synonyms |
AY 22989; AY22989; AY-22989; NSC-2260804; RAPA; RAP; RPM; SLM; AY 22989; SILA 9268A; WY090217; WY-090217; WY 090217; C07909; D00753; I 2190A; I-2190A; I2190A; NSC 226080; Rapamune
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Formulation 1: 2% DMSO + 30% PEG 300 + 5% Tween 80 + ddH2O: 5 mg/mL; suspension
Formulation 2: 0.5% CMC-Na + 1%Tween-80 in Saline water: 1.98 mg/mL (2.17 mM); suspension Formulation 3: 10% DMSO + 90% Corn Oil: ≥ 2.08 mg/mL (2.28 mM); clear solution Formulation 4: 10% EtOH + 40% PEG300 + 5% Tween-80 + 45% Saline: ≥ 2.5 mg/mL (2.73 mM); suspension Formulation 5: 10% EtOH + 90% (20% SBE-β-CD in Saline): 2.5 mg/mL (2.73 mM); suspension Formulation 6: 10% EtOH + 90% Corn Oil: ≥ 2.5 mg/mL (2.73 mM); suspension Formulation 7: 10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline: ≥ 2.08 mg/mL (2.28 mM); clear solution Formulation 8: 10% DMSO + 90% (20% SBE-β-CD in Saline): 2.08 mg/mL (2.28 mM); suspension (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0939 mL | 5.4694 mL | 10.9388 mL | |
| 5 mM | 0.2188 mL | 1.0939 mL | 2.1878 mL | |
| 10 mM | 0.1094 mL | 0.5469 mL | 1.0939 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
CD40-L Blockade for Prevention of Acute Graft-Versus-Host Disease
CTID: NCT03605927
Phase: Phase 1 Status: Completed
Date: 2024-11-27
|
 |
 |