
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
RG-2833 (formerly RGFP-109) is a potent, selective and brain-permeable inhibitor of HDAC (histone deacetylase) with potential neuroprotective effects. In cell-free assays, it inhibits HDAC1 and HDAC3 with IC50s of 60 nM and 50 nM, respectively. An experimental medication candidate called RG2833 is being researched to treat Parkinson's disease. It is being studied in phase I clinical trials after being granted orphan drug status. In an iPSC-derived neuronal cell model, FXN was upregulated and maximal deacetylase was inhibited by plasma RG2833 (5μM). The findings demonstrated a strong correlation between the downregulation of deacetylase activity and the increase in FXN (Friedreich Ataxia) transcript, indicating that deacetylation is the mechanism of action of RG2833.
| Targets |
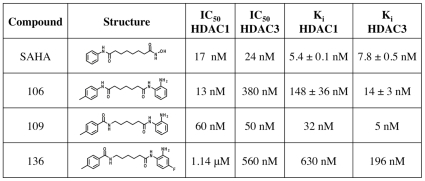
Histone Deacetylase; HDAC3 ( IC50 = 50 nM ); HDAC1 ( IC50 = 60 nM ); HDAC1 ( Ki = 32 nM ); HDAC3 ( Ki = 5 nM )
|
|---|---|
| ln Vitro |
- Frataxin Upregulation: In fibroblasts from Friedreich's ataxia patients, RG2833 (0.1–10 μM) induced dose-dependent frataxin protein expression, with a 2.5-fold increase at 1 μM. Western blot analysis confirmed upregulation of frataxin and acetylated histone H3K9. qPCR revealed a 1.8-fold increase in FXN mRNA levels after 24-hour treatment [1]
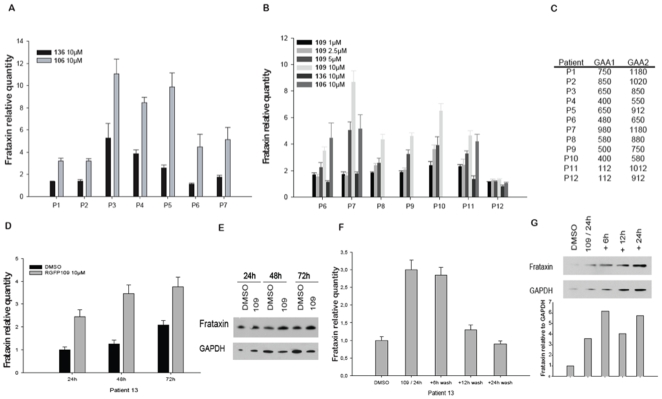
- Cell Viability: Treatment with RG2833 (1–10 μM) for 72 hours did not significantly affect cell viability in normal human fibroblasts or HEK293 cells, as assessed by MTT assay [1] - HDAC Inhibition: RG2833 potently inhibited HDAC1 and HDAC2 enzymatic activity in cell lysates, with IC50 values of 0.1 μM and 0.3 μM, respectively. Fluorometric assays using a fluorescent HDAC substrate showed dose-dependent suppression of deacetylation [1] In vitro activity: RG2833's Ki values for HDAC1 and HDAC3 are 32 nM and 5 nM, in that order. RG2833 demonstrates high activity throughout the entire tested concentration range of 1 to 10 µM. Frataxin protein increases more slowly in cells from patient P13 when RG2833 is continuously cultivated, but it increases quickly in the cells after the compound is removed[1]. In addition to reducing neuronal pathology of the dorsal root ganglia (DRG), RG2833 causes notable increases in brain aconitase enzyme activity[2]. |
| ln Vivo |
- Neuroprotective Efficacy in YG8R Mice: Oral administration of RG2833 (10 mg/kg daily for 4 weeks) significantly improved motor coordination in YG8R mice, as measured by increased latency to fall in rotarod tests. Histological analysis revealed a 30% reduction in spinal cord neuronal loss and a 2-fold increase in frataxin levels in liver and brain tissues [2]
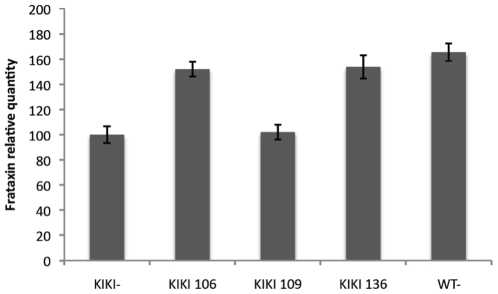
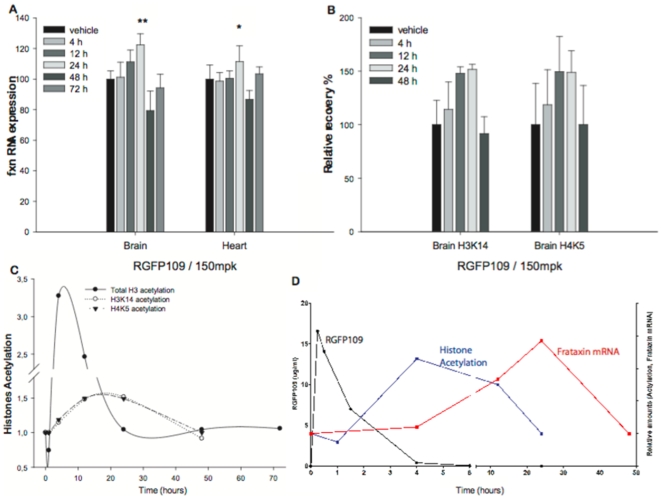
- Amelioration of L-DOPA-Induced Dyskinesia: In MPTP-lesioned marmosets, RG2833 (0.1–1 mg/kg orally) reduced dyskinesia severity by 40–60% during L-DOPA challenge. PET imaging showed restored striatal dopamine transporter binding and reduced oxidative stress markers in the substantia nigra [3] RG2833 (150 mg/kg) is capable of treating KIKI mice's brain and heart frataxin deficiency 24 hours after a single injection, but not at lower doses. When tracked over time, the KIKI mouse's increased levels of frataxin mRNA caused by RG2833 can be seen in the brain and heart at 12 hours and 24 hours, respectively[1]. After a chronic dosage of 100 mg/kg, s.c., mice tolerate RG2833 well and do not experience any toxicity. RG2833 enhances YG8R FRDA mice's motor coordination. In the brains of YG8R FRDA mice, RG2833 increases the expression of the frataxin protein[2]. After a single or six-day once-daily treatment, RGFP109 (30 mg/kg, p.o. once daily for six days) has no acute effects on dyskinesia. Dyskinesia and the amount of time spent ON-time with incapacitating dyskinesia are reduced by 37% and 50%, respectively, one week after RGFP109 is stopped[3]. Sub-chronic treatment with RGFP109 alleviates established l-DOPA-induced dyskinesia [3] Acute challenges of RGFP109 did not reduce LID severity, as shown by the lack of anti-dyskinetic efficacy on D1 and, to a certain extent, D6. However, RGFP109 significantly reduced severity of peak-dose LID and decreased duration of “bad quality” ON-time on D12, six days after cessation of treatment with RGFP109. This delayed onset of anti-dyskinetic efficacy is consistent with long-lasting changes at the nuclear level, as would be expected with HDAC inhibition, as opposed to blockade of a synaptic receptor, which would be expected to produce an immediate benefit. Importantly, the anti-dyskinetic effect of RGFP109 was obtained without compromising peak anti-parkinsonian efficacy or duration of l-DOPA benefit, suggesting that abnormal histone deacetylation is a consequence of LID and not l-DOPA therapy per se. |
| Enzyme Assay |
- HDAC1/HDAC2 Activity Assay: Recombinant HDAC1 or HDAC2 was incubated with RG2833 (0.01–10 μM) in buffer containing Tris-HCl (pH 8.0), NaCl, and DTT. After adding a fluorescent substrate (Ac-Arg-Lys-Lys-AMC), the reaction was monitored at 360 nm excitation/460 nm emission. IC50 values were calculated from dose-response curves [1]
Aconitase activities are measured by centrifuging mouse brain tissues at 800×g for 10 min at 4°C after homogenizing them on ice at 10% w/v in CellLytic MT Mammalian Tissue Lysis/Extraction buffer. After adding 50 μL of tissue lysates to 200 μL of substrate mix (which included 50 mM Tris/HCl pH7.4, 0.4 mM NADP, 5 mM Na citrate, 0.6 mM MgCl2, 0.1% (v/v) Triton X-100, and 1U isocitrate dehydrogenase), the reactions were incubated for 15 minutes at 37°C.The reaction slope was then determined by taking spectrophotometric absorbance measurements every minute for 15 minutes at 340 nm 37°C. Afterwards, using a citrate synthase assay kit, the aconitase activities of mouse brain tissues are normalized to citrate synthase activities. |
| Cell Assay |
- Frataxin Induction in Patient Cells: Friedreich's ataxia fibroblasts were treated with RG2833 (0.1–10 μM) for 24 hours. Total protein was extracted, and frataxin levels were quantified by Western blot using specific antibodies. Densitometric analysis showed a linear dose-response relationship [1]
- HDAC Activity in Cell Lysates: HEK293 cells were treated with RG2833 (0.1–10 μM) for 6 hours. Cell lysates were incubated with a fluorescent HDAC substrate, and deacetylation activity was measured using a microplate reader. The IC50 for HDAC1 inhibition was determined to be 0.1 μM [1] RG2833's Ki values for HDAC1 and HDAC3 are 5.4 nM and 7.8 nM, in that order. RG2833 demonstrates high activity throughout the entire tested concentration range of 1 to 10 µM. Frataxin protein increases more slowly in cells from patient P13 when RG2833 is continuously cultivated; however, upon removal of the compound, frataxin protein levels rose quickly. In addition to reducing neuronal pathology in the dorsal root ganglia (DRG), RG2833 causes notable increases in brain aconitase enzyme activity. |
| Animal Protocol |
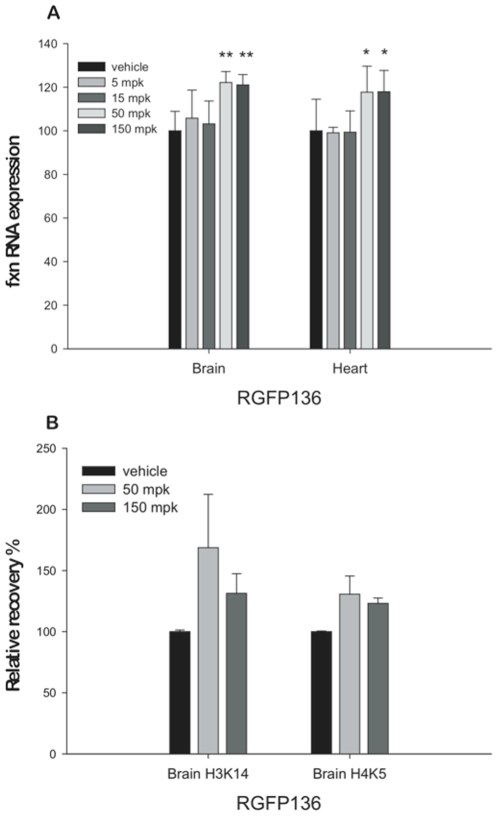
- YG8R Mouse Model: RG2833 was dissolved in 0.5% methylcellulose and administered orally to YG8R mice (10 mg/kg daily) for 4 weeks. Motor function was assessed weekly via rotarod tests. At termination, tissues were harvested for frataxin quantification and histopathological analysis [2]
- MPTP-Lesioned Marmoset Model: Marmosets received MPTP (0.3 mg/kg i.p. daily for 5 days) to induce parkinsonism. RG2833 (0.1–1 mg/kg orally) was administered 30 minutes before L-DOPA (10 mg/kg) challenges. Dyskinesia severity was scored using a validated rating scale [3] Mice are kept in standard open cages with 13 hours of light, 11 hours of darkness, 20–23°C, 45–60% humidity, Litaspen Premium 8/20 bedding, paper wool nesting, and standard fun tunnel environmental enrichment. SDS RM3 Expanded food pellets and regular drinking water are fed to the mice. The mice are subcutaneously injected with 150 mg/kg RG2833 three times a week for 4.5 months, or 50 mg/kg 136 or 100 mg/kg RG2833 five times a week for five months. Twenty-four hours after the last injection, the mice are culled in order to collect tissue. Administration of RG2833 (RGFP109) in combination with l-DOPA to the parkinsonian marmoset [3] A schematic depicting the time line of the experiments conducted is provided in Fig. 2. Twelve days prior to the start of the study (D-12), animals were administered an acute challenge of l-DOPA/benserazide (20/5 mg/kg s.c., henceforth referred to as l-DOPA). Behaviour observed on D-12 was used as a baseline comparator to ensure that the animals responded consistently to l-DOPA, both in terms of dyskinesia and duration of reversal of parkinsonism, thereby ensuring that changes noted throughout the study would be secondary to HDAC inhibition and not variation in the response to l-DOPA. On study day 0 (D0), animals were treated with an acute challenge of l-DOPA in combination with vehicle. Treatment with the HDACi RG2833 (RGFP109) was initiated 24 h later (D1). Throughout the study, animals were administered RG2833 (RGFP109) orally (30 mg/kg) dissolved in hydroxypropyl-β-cyclodextrin acetate (50%, v/v) in water, in combination with l-DOPA. Both drugs were administered simultaneously. The dose of l-DOPA was kept constant throughout the observation days (20/5 mg/kg), but was administered orally on non-behavioural days and s.c. on behavioural observation days (D-12, D0, D1, D6 and D12), in order to minimise variability due to erratic gastro-intestinal absorption. Treatment with RG2833 (RGFP109) was ceased on D6. After a six-day wash-out period during which daily l-DOPA treatment was maintained, response to an acute l-DOPA challenge was re-assessed (D12). |
| ADME/Pharmacokinetics |
- Oral Bioavailability: In mice, RG2833 demonstrated moderate oral bioavailability (25%) with peak plasma concentrations (Cmax) of 0.8 μg/mL achieved within 1 hour. The compound showed high plasma protein binding (>90%) and a terminal half-life of 4–6 hours [2]
- Tissue Distribution: After oral administration, RG2833 accumulated in the brain, achieving brain/plasma concentration ratios of 0.6–0.8. Significant levels were also detected in liver and skeletal muscle [2] |
| Toxicity/Toxicokinetics |
Oral RGFP109 treatment was well-tolerated with no adverse effects observed throughout the study. Over the twelve-day study period, there was a significant effect of treatment on levels of LID during periods of peak l-DOPA effect (Friedman statistic (FS) = 9.75, P < 0.01, Friedman test, Fig. 3B). However, neither acute (D1) nor six days (D6) of treatment with RGFP109 co-administered with l-DOPA, had any effect on levels of LID compared to l-DOPA alone on D0 (all P > 0.05, Dunn's post hoc test). On D12, 6 days after cessation of RGFP109 treatment, although daily l-DOPA was continued, there was a significant reduction (by 37%) in levels of LID (21.5 ± 1.0 on D0 and 13.5 ± 1.5 on D12; P < 0.05, Dunn's post hoc test, Fig. 3B). Accordingly, across the study, there was a significant effect of treatment on the duration of ON-time with disabling dyskinesia (F3,9 = 5.6, P < 0.05, one-way RM ANOVA). At D12, but not prior to this point, the duration of ON-time with disabling dyskinesia was reduced by 50% compared to l-DOPA alone on D0 (145 ± 11 min on D0 and 73 ± 23 min on D12; P < 0.05, Tukey's post hoc test Fig. 3F). [3]
- Acute Toxicity: The oral LD50 of RG2833 in mice exceeded 1000 mg/kg. No mortality or severe adverse effects were observed in acute toxicity studies [1,2] - Chronic Toxicity: In a 4-week oral toxicity study in rats, RG2833 (20 mg/kg daily) caused no significant changes in hematology, serum biochemistry, or organ weights. Mild reversible liver hypertrophy was noted at the highest dose [2] - Drug-Drug Interactions: Co-administration with ketoconazole (a CYP3A4 inhibitor) increased RG2833 plasma levels by 2.3-fold, indicating potential pharmacokinetic interactions [2] |
| References |
|
| Additional Infomation |
Background: Friedreich's ataxia (FRDA), the most common recessive ataxia in Caucasians, is due to severely reduced levels of frataxin, a highly conserved protein, that result from a large GAA triplet repeat expansion within the first intron of the frataxin gene (FXN). Typical marks of heterochromatin are found near the expanded GAA repeat in FRDA patient cells and mouse models. Histone deacetylase inhibitors (HDACIs) with a pimelic diphenylamide structure and HDAC3 specificity can decondense the chromatin structure at the FXN gene and restore frataxin levels in cells from FRDA patients and in a GAA repeat based FRDA mouse model, KIKI, providing an appealing approach for FRDA therapeutics.
Methodology/principal findings: In an effort to further improve the pharmacological profile of pimelic diphenylamide HDACIs as potential therapeutics for FRDA, we synthesized additional compounds with this basic structure and screened them for HDAC3 specificity. We characterized two of these compounds, 136 and 109, in FRDA patients' peripheral blood lymphocytes and in the KIKI mouse model. We tested their ability to upregulate frataxin at a range of concentrations in order to determine a minimal effective dose. We then determined in both systems the duration of effect of these drugs on frataxin mRNA and protein, and on total and local histone acetylation. The effects of these compounds exceeded the time of direct exposure in both systems.
Conclusions/significance: Our results support the pre-clinical development of a therapeutic approach based on pimelic diphenylamide HDACIs for FRDA and provide information for the design of future human trials of these drugs, suggesting an intermittent administration of the drug. [1]
Friedreich ataxia (FRDA) is an inherited neurodegenerative disorder caused by GAA repeat expansion within the FXN gene, leading to epigenetic changes and heterochromatin-mediated gene silencing that result in a frataxin protein deficit. Histone deacetylase (HDAC) inhibitors, including pimelic o-aminobenzamide compounds 106, 109 and 136, have previously been shown to reverse FXN gene silencing in short-term studies of FRDA patient cells and a knock-in mouse model, but the functional consequences of such therapeutic intervention have thus far not been described. We have now investigated the long-term therapeutic effects of 106, 109 and 136 in our GAA repeat expansion mutation-containing YG8R FRDA mouse model. We show that there is no overt toxicity up to 5 months of treatment and there is amelioration of the FRDA-like disease phenotype. Thus, while the neurological deficits of this model are mild, 109 and 106 both produced an improvement of motor coordination, whereas 109 and 136 produced increased locomotor activity. All three compounds increased global histone H3 and H4 acetylation of brain tissue, but only 109 significantly increased acetylation of specific histone residues at the FXN locus. Effects on FXN mRNA expression in CNS tissues were modest, but 109 significantly increased frataxin protein expression in brain tissue. 109 also produced significant increases in brain aconitase enzyme activity, together with reduction of neuronal pathology of the dorsal root ganglia (DRG). Overall, these results support further assessment of HDAC inhibitors for treatment of Friedreich ataxia. [2] Background: l-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesia (LID) are a complication of chronic dopamine replacement therapy in Parkinson's disease (PD). Recent studies have suggested that the mechanisms underlying development and expression of LID in PD may involve epigenetic changes that include deacetylation of striatal histone proteins. We hypothesised that inhibition of histone deacetylase, the enzyme responsible of histone deacetylation, would alleviate LID. Methods: Four female common marmoset (Callithrix jacchus) were rendered parkinsonian by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Following stabilisation of the parkinsonian phenotype, marmosets were primed to exhibit dyskinesia with chronic administration of L-DOPA. We then investigated the effects of the brain-penetrant histone deacetylase inhibitor, RGFP109 (30 mg/kg p.o. once daily for 6 days), on LID and L-DOPA anti-parkinsonian efficacy. Results: RGFP109 had no acute effects on dyskinesia after single or 6 days once-daily treatment (both P > 0.05). However, one week following cessation of RGFP109, dyskinesia and duration of ON-time with disabling dyskinesia were reduced by 37% and 50%, respectively (both P < 0.05), compared to that seen previously with L-DOPA alone. There was no change in anti-parkinsonian actions of, or ON-time duration afforded by, L-DOPA (P > 0.05). Conclusions: Histone deacetylation inhibition may represent a novel approach to reverse established LID in PD and improve quality of the anti-parkinsonian benefit provided by L-DOPA. [3] - Mechanism of Action: RG2833 selectively inhibits HDAC1/HDAC2, leading to increased acetylation of histone H3K9 and transcriptional activation of the FXN gene. This restores frataxin levels, improving mitochondrial iron-sulfur cluster biosynthesis and reducing oxidative stress [1,2] - Therapeutic Potential: Beyond Friedreich's ataxia and Parkinson’s disease, RG2833 is being evaluated for Huntington’s disease and amyotrophic lateral sclerosis (ALS) in preclinical models [2,3] - Clinical Development: RG2833 has completed Phase I trials in healthy volunteers, demonstrating a favorable safety profile. Phase II trials for Friedreich's ataxia are ongoing [3] |
| Molecular Formula |
C20H25N3O2
|
|
|---|---|---|
| Molecular Weight |
339.43
|
|
| Exact Mass |
339.195
|
|
| Elemental Analysis |
C, 70.77; H, 7.42; N, 12.38; O, 9.43
|
|
| CAS # |
1215493-56-3
|
|
| Related CAS # |
|
|
| PubChem CID |
56654642
|
|
| Appearance |
White to off-white solid powder
|
|
| LogP |
5.127
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
3
|
|
| Rotatable Bond Count |
8
|
|
| Heavy Atom Count |
25
|
|
| Complexity |
419
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])N([H])C(C1C([H])=C([H])C(C([H])([H])[H])=C([H])C=1[H])=O)N([H])C1=C([H])C([H])=C([H])C([H])=C1N([H])[H]
|
|
| InChi Key |
VOPDXHFYDJAYNS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H25N3O2/c1-15-10-12-16(13-11-15)20(25)22-14-6-2-3-9-19(24)23-18-8-5-4-7-17(18)21/h4-5,7-8,10-13H,2-3,6,9,14,21H2,1H3,(H,22,25)(H,23,24)
|
|
| Chemical Name |
N-[6-(2-aminoanilino)-6-oxohexyl]-4-methylbenzamide
|
|
| Synonyms |
RGFP 109; RGFP-109; RGFP109; RG2833; RG2833; 1215493-56-3; N-(6-((2-Aminophenyl)amino)-6-oxohexyl)-4-methylbenzamide; RGFP-109; N-(6-(2-Aminophenylamino)-6-oxohexyl)-4-methylbenzamide; RGFP109; RG 2833; RG-2833
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.37 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.37 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (7.37 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: ≥ 2.5 mg/mL (7.37 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: 5%DMSO Corn oil: 6.0mg/ml (17.68mM) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9461 mL | 14.7306 mL | 29.4612 mL | |
| 5 mM | 0.5892 mL | 2.9461 mL | 5.8922 mL | |
| 10 mM | 0.2946 mL | 1.4731 mL | 2.9461 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
  |
|---|
  |
 |