
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Saracatinib (formerly also known as AZD-0530; AZD0530) is a novel, potent Src kinase inhibitor with potential antineoplastic activity. It inhibits Src with an IC50 of 2.7 nM in cell-free assays, and also potently inhibits other kinases such as c-Yes, Fyn, Lyn, Blk, Fgr and Lck; Saracatinib is less active against Abl and EGFR (L858R and L861Q). Saracatinib exhibits excellent in vivo antitumor efficacy in various tumors such as Src3T3 allografts, DU145, Calu-6, MDA-MB-231, AsPc-1 and BT474C xenografts. It has been reported to inhibit Src activation in DU145 and PC3 cell lines (prostate cancer cell lines). Both of c-Myc and cyclin D1 expression are decreased by Saracatinib. Saracatinib can inhibit the ERK1/2 and GSK3b phosphorylation as well as decrease β-catenin level in cells. Saracatinib inhibits the prostate tumor cell growth by inducing cycle arrest at G1/S phase. Saracatinib dose-dependently blocks cell migration in DU145 and PC3 cell lines.
| ln Vitro |
An oral Src inhibitor called Saracatinib (AZD0530) has been shown to have strong antimigratory and anti-invasive properties in vitro. It also prevents bladder cancer metastases in a mouse model. Saracatinib's antiproliferative efficacy varies between cell lines (IC50 0.2-10 μM). Saracatinib exhibits varying antiproliferative efficacy in a variety of human cancer cell lines expressing endogenous Src and potently inhibits the proliferation of Src3T3 murine fibroblasts. IC50 values of 0.2-0.7 μM are obtained for five human cancer cell lines examined with Saracatinib, representing the tumor types colon, prostate, lung, and leukemia, indicating submicromolar growth suppression. Saracatinib suppresses the Bcr-Abl-driven human leukemia cell line K562's proliferation in 3-day MTS cell proliferation tests, with an IC50 of 0.22 μM. Saracatinib decreases human lung cancer A549 cells' movement in the microdroplet migration assay in a concentration-dependent manner (IC50 0.14 μM)[1].
|
||
|---|---|---|---|
| ln Vivo |
Treatment with Saracatinib (AZD0530) potently and dose-dependently suppresses the proliferation of transplanted Src3T3 fibroblasts under the skin in mice and rats. Significant tumor growth inhibition is observed in both models at doses ≥6 mg/kg/day (60% in mice and 98% in rats versus animals treated with vehicle), and complete tumor growth inhibition is observed at the highest doses investigated (100% in rats and mice at 25 mg/kg/day and 10 mg/kg/day, respectively) [1].
|
||
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
Saracatinib is a member of the class of quinazolines that is quinazoline substituted by (5-chloro-2H-1,3-benzodioxol-4-yl)amino, (oxan-4-yl)oxy and 2-(4-methylpiperazin-1-yl)ethoxy groups at positions 4, 5 and 7, respectively. It is a dual inhibitor of the tyrosine kinases c-Src and Abl (IC50 = 2.7 and 30 nM, respectively). Saracatinib was originally developed by AstraZeneca for the treatment of cancer but in 2019 it was granted orphan drug designation by the US Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (IPF), a type of lung disease that results in scarring (fibrosis) of the lungs. It has a role as an antineoplastic agent, an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, a radiosensitizing agent, an autophagy inducer, an apoptosis inducer and an anticoronaviral agent. It is a member of quinazolines, a secondary amino compound, a N-methylpiperazine, an aromatic ether, a member of oxanes, a member of benzodioxoles, an organochlorine compound and a diether.
Saracatinib has been investigated for the treatment of Cancer, Osteosarcoma, Ovarian Cancer, Fallopian Tube Cancer, and Primary Peritoneal Cancer. Saracatinib is an orally available 5-, 7-substituted anilinoquinazoline with anti-invasive and anti-tumor activities. Saracatinib is a dual-specific inhibitor of Src and Abl, protein tyrosine kinases that are overexpressed in chronic myeloid leukemia cells. This agent binds to and inhibits these tyrosine kinases and affects cell motility, cell migration, adhesion, invasion, proliferation, differentiation, and survival. Specifically, Saracatinib inhibits Src kinase-mediated osteoclast bone resorption. |
| Molecular Formula |
C27H32CLN5O5
|
|---|---|
| Molecular Weight |
542.03
|
| Exact Mass |
541.209
|
| CAS # |
379231-04-6
|
| Related CAS # |
Saracatinib difumarate;893428-72-3
|
| PubChem CID |
10302451
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
671.3±55.0 °C at 760 mmHg
|
| Flash Point |
359.8±31.5 °C
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
| Index of Refraction |
1.641
|
| LogP |
2.74
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
38
|
| Complexity |
743
|
| Defined Atom Stereocenter Count |
0
|
| InChi Key |
OUKYUETWWIPKQR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31)
|
| Chemical Name |
N-(5-Chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methyl-1-piperazinyl)ethoxy]-5-[(tetrahydro-2H-pyran-4-yl)oxy]-4-quinazolinamine
|
| Synonyms |
AZD0530; Saracatinib; AZD-0530; AZD 0530
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 2% DMSO+30% PEG 300+ddH2O: 5 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8449 mL | 9.2246 mL | 18.4492 mL | |
| 5 mM | 0.3690 mL | 1.8449 mL | 3.6898 mL | |
| 10 mM | 0.1845 mL | 0.9225 mL | 1.8449 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04307953 | Recruiting | Drug: AZD0530 Difumarate Drug: Matching placebo |
Fibrodysplasia Ossificans Progressiva | Amsterdam UMC, location VUmc | August 5, 2020 | Phase 2 |
| NCT02116712 | Completed | Drug: Saracatinib | Pulmonary Lymphangioleiomyomatosis | Tony Eissa | August 2014 | Phase 1 |
| NCT02732587 | Completed Has Results | Drug: Saracatinib | Alcohol Drinking | Yale University | November 2015 | Phase 1 |
| NCT02737202 | Terminated | Drug: saracatinib | Pulmonary Lymphangioleiomyomatosis | Baylor College of Medicine | April 2016 | Phase 2 |
|
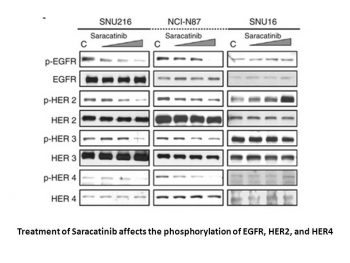 |
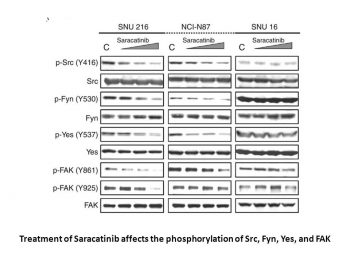 |
AZD0530 inhibits cell proliferation through β-catenin, ERK1/2 and GSK3β-mediated cyclin D1 and c-myc regulation.Oncogene.2008 Oct 23;27(49):6365-75. |
AZD0530 inhibits Src activation through inhibition of Y419 phosphorylation.Oncogene.2008 Oct 23;27(49):6365-75. |
AZD0530 inhibits cell migration through Src-mediated FAK activation.Oncogene.2008 Oct 23;27(49):6365-75. |