
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
Purity: = 100%
SB-334867 is a novel, non-peptide, selective orexin-1 (OX1) receptor antagonist with a pKb value of 7.2. It was the first non-peptide antagonist developed that is selective for the orexin receptor subtype OX1, with around 50x selectivity for OX1 over OX2 receptors. It has been demonstrated to have sedative and anorectic effects in animals. It has also proven helpful in defining how orexinergic regulation of brain systems related to appetite, sleep, and other physiological processes is regulated.
| Targets |
OX1 receptor
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
SB-334867 free base has an IC50 value of 7.2 (pKb) and is a selective non-peptide orexin OX1 receptor antagonist. In CHO-OX(1) cells, SB-334867-A did not affect the calcium response induced by UTP (3 microM) but it did suppress the responses to orexin-A (10 nM) and orexin-B (100 nM) (pK(B)=7.27+/-0.04 and 7.23+/-0.03, respectively, n=8). The calcium responses mediated by OX(2) were also inhibited by SB-334867-A (10 microM) (32.7+/-1.9% versus orexin-A).
|
||
| Cell Assay |
Two peptides extracted from the rat hypothalamus are called orexin-A and orexin-B. Some physiological processes that they are involved in include feeding regulation, energy metabolism, and sleep-wake cycle regulation. In CHO-OX1 cells, SB-334867 can suppress the calcium responses induced by orexin-A and orexin-B, with pKB values of 7.27 and 7.23, respectively. For OX1 receptors, SB-334867 exhibits greater selectivity than OX2 receptors. It inhibits the calcium responses in CHO-OX2 cells that are induced by orexin-A by 32.7% and orexin-B by 22%, respectively.
|
||
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
1-(2-methyl-1,3-benzoxazol-6-yl)-3-(1,5-naphthyridin-4-yl)urea is a naphthyridine derivative.
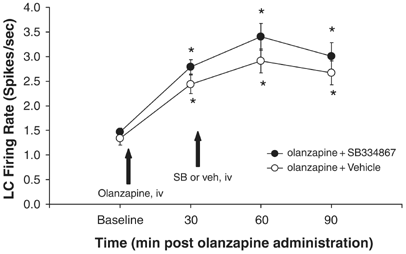
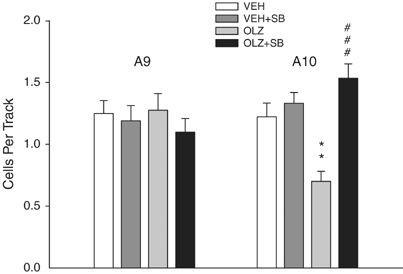
The present study focused upon the role of SB-334867, an orexin-1 receptor antagonist, in the acquisition of morphine-induced sensitization to locomotor activity in mice. Behavioral sensitization is an enhanced systemic reaction to the same dose of an addictive substance, which assumingly increases both the desire for the drug and the risk of relapse to addiction. Morphine-induced sensitization in mice was achieved by sporadic doses (five injections every 3 days) of morphine (10 mg/kg, i.p.), while a challenge dose of morphine (10 mg/kg) was injected 7 days later. In order to assess the impact of orexin system blockade on the acquisition of sensitization, SB-334867 was administered before each morphine injection, except the morphine challenge dose. The locomotor activity test was performed on each day of morphine administration. Brain structures (striatum, hippocampus, and prefrontal cortex) were collected after behavioral tests for molecular experiments in which mRNA expression of orexin, dopamine, and adenosine receptors was explored by the qRT-PCR technique. Additionally, the mRNA expression of markers, such as GFAP and Iba-1, was also analyzed by the same technique. SB-334867 inhibited the acquisition of morphine-induced sensitization to locomotor activity of mice. Significant alterations were observed in mRNA expression of orexin, dopamine, and adenosine receptors and in the expression of GFAP and Iba-1, showing a broad range of interactions in the mesolimbic system among orexin, dopamine, adenosine, and glial cells during behavioral sensitization. Summing up, the orexin system may be an effective measure to inhibit morphine-induced behavioral sensitization.[1] To examine the role of orexin-1 and orexin-2 receptor activity on ethanol self-administration, compounds that differentially target orexin (OX) receptor subtypes were assessed in various self-administration paradigms using high-drinking rodent models. Effects of the OX1 antagonist SB334867, the OX2 antagonist LSN2424100, and the mixed OX1/2 antagonist almorexant (ACT-078573) on home cage ethanol consumption were tested in ethanol-preferring (P) rats using a 2-bottle choice procedure. In separate experiments, effects of SB334867, LSN2424100, and almorexant on operant ethanol self-administration were assessed in P rats maintained on a progressive ratio operant schedule of reinforcement. In a third series of experiments, SB334867, LSN2424100, and almorexant were administered to ethanol-preferring C57BL/6J mice to examine effects of OX receptor blockade on ethanol intake in a binge-like drinking (drinking-in-the-dark) model. In P rats with chronic home cage free-choice ethanol access, SB334867 and almorexant significantly reduced ethanol intake, but almorexant also reduced water intake, suggesting non-specific effects on consummatory behavior. In the progressive ratio operant experiments, LSN2424100 and almorexant reduced breakpoints and ethanol consumption in P rats, whereas the almorexant inactive enantiomer and SB334867 did not significantly affect the motivation to consume ethanol. As expected, vehicle-injected mice exhibited binge-like drinking patterns in the drinking-in-the-dark model. All three OX antagonists reduced both ethanol intake and resulting blood ethanol concentrations relative to vehicle-injected controls, but SB334867 and LSN2424100 also reduced sucrose consumption in a different cohort of mice, suggesting non-specific effects. Collectively, these results contribute to a growing body of evidence indicating that OX1 and OX2 receptor activity influences ethanol self-administration, although the effects may not be selective for ethanol consumption.[2] The pharmacology of various peptide and non-peptide ligands was studied in Chinese hamster ovary (CHO) cells stably expressing human orexin-1 (OX(1)) or orexin-2 (OX(2)) receptors by measuring intracellular calcium ([Ca(2+)](i)) using Fluo-3AM. Orexin-A and orexin-B increased [Ca(2+)](i) in CHO-OX(1) (pEC(50)=8.38+/-0.04 and 7.26+/-0.05 respectively, n=12) and CHO-OX(2) (pEC(50)=8.20+/-0.03 and 8.26+/-0.04 respectively, n=8) cells. However, neuropeptide Y and secretin (10 pM - 10 microM) displayed neither agonist nor antagonist properties in either cell-line. SB-334867-A (1-(2-Methyylbenzoxanzol-6-yl)-3-[1,5]naphthyridin-4-yl-urea hydrochloride) inhibited the orexin-A (10 nM) and orexin-B (100 nM)-induced calcium responses (pK(B)=7.27+/-0.04 and 7.23+/-0.03 respectively, n=8), but had no effect on the UTP (3 microM)-induced calcium response in CHO-OX(1) cells. SB-334867-A (10 microM) also inhibited OX(2) mediated calcium responses (32.7+/-1.9% versus orexin-A). SB-334867-A was devoid of agonist properties in either cell-line. In conclusion, SB-334867-A is a non-peptide OX(1) selective receptor antagonist.[3] |
| Molecular Formula |
C17H13N5O2
|
|
|---|---|---|
| Molecular Weight |
319.32
|
|
| Exact Mass |
319.106
|
|
| Elemental Analysis |
C, 63.94; H, 4.10; N, 21.93; O, 10.02
|
|
| CAS # |
792173-99-0
|
|
| Related CAS # |
SB-334867; 249889-64-3
|
|
| PubChem CID |
6604926
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Boiling Point |
549.5±58.0 °C at 760 mmHg
|
|
| Flash Point |
286.1±32.3 °C
|
|
| Vapour Pressure |
0.0±1.5 mmHg at 25°C
|
|
| Index of Refraction |
1.757
|
|
| LogP |
0.51
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
2
|
|
| Heavy Atom Count |
24
|
|
| Complexity |
462
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(NC1C2C(=CC=CN=2)N=CC=1)NC1C=C2C(N=C(C)O2)=CC=1
|
|
| InChi Key |
AKMNUCBQGHFICM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H13N5O2/c1-10-20-12-5-4-11(9-15(12)24-10)21-17(23)22-14-6-8-18-13-3-2-7-19-16(13)14/h2-9H,1H3,(H2,18,21,22,23)
|
|
| Chemical Name |
1-(2-methyl-1,3-benzoxazol-6-yl)-3-(1,5-naphthyridin-4-yl)urea
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.83 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (7.83 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (7.83 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 1% CMC Na : 30mg/mL Solubility in Formulation 5: 10 mg/mL (31.32 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 6: 7.69 mg/mL (24.08 mM) in 50% HP-β-CD in Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1317 mL | 15.6583 mL | 31.3165 mL | |
| 5 mM | 0.6263 mL | 3.1317 mL | 6.2633 mL | |
| 10 mM | 0.3132 mL | 1.5658 mL | 3.1317 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
 |