
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
Purity: ≥98%
Adezmapimod (SB-203580; RWJ-64809; SB203580; RWJ 64809) is a novel and potent p38 mitogen-activated protein kinase inhibitor (p38MAPK inhibitor) that has the potential to treat Systemic lupus erythematosus (SLE). In THP-1 cells, it blocks PKB phosphorylation with an IC50 of 3-5 μM, inhibits p38MAPK with IC50s of 0.3-0.5 μM , and is 10-fold less potent than SAPK3(106T) and SAPK4(106T). By reducing proinflammatory cytokines and proteolytic factors in a mouse model, SB203580 inhibits the growth of endometriosis. With a Ki of 21 nM, SB203580 is a competitive ATPsite inhibitor of p38MAPK with selectivity likely influenced by nonconserved regions within or close to the ATP binding pocket.
| Targets |
p38 (IC50 = 50 nM); p38β2 (IC50 = 500 nM)
|
|---|---|
| ln Vitro |
SB203580 has an IC50 of 3-5 μm and inhibits the proliferation of murine CT6 T cells, BAF F7 B cells, or primary human T cells when IL-2 is present. Although the concentration needed is a little bit higher and the IC50 is above 10 μm, SB203580 also inhibits IL-2-induced p70S6 kinase activation. With an IC50 in the 3-10 μm range, SB203580 also inhibits the activity of PDK1 in a dose-dependent manner.[1] SB203580 has an IC50 of about 0.07 μm for blocking p38-MAPK stimulation of MAPKAPK2, whereas it has an IC50 of 3–10 μm for blocking total SAPK/JNK activity. Higher concentrations of SB203580 cause the ERK pathway to be activated, which then improves the transcriptional activity of NF-κB.[2] Human hepatocellular carcinoma (HCC) cells are induced to undergo autophagy by SB203580.[3]
|
| ln Vivo |
SB203580 protects pig myocardium in an in vivo model from ischemic damage.[4] SB203580 is effective at both preventing and treating Systemic Lupus Erythematosus (SLE) in MRL/lpr mice.[5]
Proteinuria is prevented in SB203580 treated MRL/lpr mice. [5] ALT and AST are not influenced by SB203580 in MRL/lpr mice. [5] BUN but not Cr is decreased in SB203580 treated MRL/lpr mice. Renal but not splenic weight is reduced in SB203580 treated MRL/lpr mice. [5] Renal pathologic changes are attenuated in SB203580 treated MRL/lpr mice. [5] Hepatic pathologic changes are relieved in SB203580 treated MRL/lpr mice. [5] Splenic pathologic changes are relieved in SB203580 treated MRL/lpr mice. [5] Glomerular IgG, IgM, IgA and C3 depositions are reduced in SB203580 treated MRL/lpr mice. [5] |
| Enzyme Assay |
Cellular receptor kinase phosphorylation assay: 4μg of sheep anti-PKBα is immobilized on 25 μL of protein G-Sepharose overnight (or 1.5 hours) and washed in Buffer A (50 mm Tris, pH 7.5, 1 mm EDTA, 1 mm EGTA, 0.5 mm Na3VO4, 0.1% β-mercaptoethanol, 1% Triton X-100, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 0.1 mm phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, pepstatin, leupeptin, and 1 μm microcystin). The immobilized anti-PKB is then incubated with 0.5 ml of the lysate (from 5 × 106 cells) for 1.5 hours, washed five times in 0.5 mL of Buffer A supplemented with 0.5 m NaCl, twice in 0.5 mL of Buffer B (50 mm Tris-HCl, pH 7.5, 0.03% (w/v) Brij-35, 0.1 mm EGTA, and 0.1% β-mercaptoethanol), and twice with 100 μl of assay dilution buffer; 5× assay dilution buffer is 100 mm MOPS, pH 7.2, 125 mm β-glycerophosphate, 25 mm EGTA, 5 mm sodium orthovanadate, 5 mm DTT. The PKB enzyme immune complex is supplemented with 10 μL of assay dilution buffer, 40 μm of protein kinase A inhibitor peptide, 100 μm of PKB-specific substrate peptide, and 10 μCi of [γ-32P]ATP. The reaction is allowed to proceed for 20 minutes at room temperature while being shaken, after which the samples are pulse spun and 40 μL of the reaction volume are transferred to another tube into which 20 μL of 40% trichloroacetic acid is added to stop the reaction. After mixing and incubating at room temperature for 5 minutes, 40 μL of the mixture is transferred onto P81 phosphocellulose paper and allowed to bind for 30 seconds. The P81 piece is cleaned in acetone at room temperature after being washed three times in 0.75% phosphoric acid. The incorporation of γ-32P is then quantified using scintillation counting.
|
| Cell Assay |
In order to rest CT6 cells and BA/F3 F7 cells, they are washed three times in RPMI and cultured for an overnight period in RPMI with 5% fetal calf serum without the addition of growth factors, antibiotics, or β-mercaptoethanol. Preincubation with SB203580 or a vehicle control is carried out on 2 mL of RPMI, 5% fetal calf serum and 2–5 × 106 rested CT6 cells, as shown in the figure legends. Afterward, cells are stimulated for 5 minutes at 37 °C with 20 ng/ml recombinant human IL-2, pelleted in a minifuge for 30 seconds, the medium is aspirated, and the pellet is lysed in the proper buffer. BA/F3 cells are maintained in glutamine-containing RPMI that is additionally supplemented with 5% fetal calf serum and 0.2 μg/mL G418 and stably express deletion mutants of the IL-2 receptor β chain. The cells are then thoroughly washed, allowed to rest for the night, and then washed once more before being activated with IL-2. Such cell preparations contain >90% T cells. The incorporation of [3H]thymidine is measured in cellular proliferation assays.
|
| Animal Protocol |
Systemic lupus erythematosus (SLE) are established in female MRL/lpr mice and female C57BL/6 mice
0.1 M/day Orally administered Female MRL/lpr mice were randomized into two groups (n = 10 per group) and were fed control diet (named as group 2 in the following) or diet with SB203580 (named as group 3 in the following) starting at the age of 14 weeks and continuing for up to 22 weeks. Adezmapimod (SB203580) was dissolved in drinking water (250 μmol/L), was orally administered (0.4 ml/day). Ten C57BL/6 female mice were used as negative controls (named as group 1 in the following). Two mice in MRL/lpr group 2 were dead at 16 weeks and 18 weeks of age respectively. Two mice in MRL/lpr group 3 were dead at 19 weeks of age. Significant increase of urine protein (300–2000 mg/dl) was found in each mouse before death, indicating a probable renal failure be the cause of death. Ultimately, 10 mice in group 1, 8 mice in group 2 and group 3 were included in statistical analysis.[5] Systemic lupus erythematosus (SLE) is an autoimmune disease accompanying excessive inflammatory responses in a wide range of organs. Abnormal activation of p38 MAPK has been postulated to contribute to the inflammation of SLE, leading to progressive tissue and organ damages to develop lupus nephritis and autoimmune hepatitis. In order to determine whether p38 MAPK inhibitor is effective in mouse model of SLE, a specific inhibitor of p38 MAPK Adezmapimod (SB203580) was orally administrated to MRL/lpr mice aged from 14 to 22 weeks. Renal and hepatic functions, as well as pathologic changes of important organs including kidney, liver and spleen of MRL/lpr mice were evaluated. As a result, we showed that SB203580 improved renal function by decreasing the levels of proteinuria and serum BUN, ameliorating the pathologic changes of kidney and reducing Ig and C(3) depositions in the kidney. Hepatocytes necrosis, recruitment and proliferation of leucocytes in liver and spleen were found to be inhibited by administration of SB203580. Therefore, p38 MAPK activation may be partially responsible for escalating autoimmune renal, hepatic and splenic destruction, and its inhibitor may lighten the autoimmune attack in these important organs and improve renal function. Our study reveals that the selective blockade of p38 MAPK is effective to prevent and treat the disease in this model of SLE.[5] |
| References | |
| Additional Infomation |
SB 203580 is a member of the class of imidazoles carrying 4-methylsulfinylphenyl, 4-pyridyl and 4-fluorophenyl substituents at positions 2, 4 and 5 respectively. An inhibitor of mitogen-activated protein kinase. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, a Hsp90 inhibitor, a neuroprotective agent, an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and a geroprotector. It is a member of imidazoles, a member of monofluorobenzenes, a member of pyridines and a sulfoxide.
4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole has been reported in Annulohypoxylon truncatum, Eleutherococcus divaricatus, and other organisms with data available. Pyridinyl imidazole inhibitors, particularly SB203580, have been widely used to elucidate the roles of p38 mitogen-activated protein (MAP) kinase (p38/HOG/SAPKII) in a wide array of biological systems. Studies by this group and others have shown that SB203580 can have antiproliferative activity on cytokine-activated lymphocytes. However, we recently reported that the antiproliferative effects of SB203580 were unrelated to p38 MAP kinase activity. This present study now shows that SB203580 can inhibit the key cell cycle event of retinoblastoma protein phosphorylation in interleukin-2-stimulated T cells. Studies on the proximal regulator of this event, the phosphatidylinositol 3-kinase/protein kinase B (PKB)(Akt/Rac) kinase pathway, showed that SB203580 blocked the phosphorylation and activation of PKB by inhibiting the PKB kinase, phosphoinositide-dependent protein kinase 1. The concentrations of SB203580 required to block PKB phosphorylation (IC(50) 3-5 microM) are only approximately 10-fold higher than those required to inhibit p38 MAP kinase (IC(50) 0.3-0.5 microM). These data define a new activity for this drug and would suggest that extreme caution should be taken when interpreting data where SB203580 has been used at concentrations above 1-2 microM.[1] In the present study we investigated a possible role for the p38 mitogen-activated protein (MAP) kinase pathway in mediating nuclear factor-kappa B (NF-kappaB) transcriptional activity in the erythroleukaemic cell line TF-1. TF-1 cells stimulated with the phosphatase inhibitor okadaic acid (OA) demonstrated enhanced NF-kappaB and GAL4p65-regulated transcriptional activity which was associated with elevated p38 phosphorylation. However, pretreatment with the p38 MAPK specific inhibitor SB203580 (1 microM) or overexpression of kinase-deficient mutants of MKK3 or MKK6 did not affect OA-enhanced NF-kappaB transcriptional potency, as determined in transient transfection assays. In fact, 5 and 10 microM SB203580 enhanced rather than inhibited NF-kappaB-mediated promoter activity by 2 fold, which was independent of phosphorylation of the p65 subunit. The SB203580-mediated increase in NF-kappaB transcriptional activity was associated with enhanced phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK), but not p38 kinase. Overexpression of kinase-deficient mutants belonging to the ERK1/2, JNK, and p38 pathways showed that only dominant-negative Raf-1 abrogated SB203580-enhanced NF-kappaB activity. This would implicate the involvement of the ERK1/2 pathway in the enhancing effects of SB203580 on NF-kappaB-mediated gene transcription. This study demonstrates that the p38 MAP kinase pathway is not involved in the OA-induced activation of NF-kappaB. SB203580 at higher concentrations activates the ERK pathway, which subsequently enhances NF-kappaB transcriptional activity.[2] SB203580 is a well-known inhibitor of p38 mitogen-activated protein kinase (MAPK). However, it can suppress cell proliferation in a p38 MAPK independent manner. The inhibitory mechanism remains unknown. Here, we showed that SB203580 induced autophagy in human hepatocellular carcinoma (HCC) cells. SB203580 increased GFP-LC3-positive cells with GFP-LC3 dots, induced accumulation of autophagosomes, and elevated the levels of microtubule-associated protein light chain 3 and Beclin 1. It stimulated the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and p53, but inhibited the phosphorylation of death-associated protein kinase (DAPK). Inhibition of AMPK, p53, or DAPK attenuated SB203580-induced autophagy. AMPK activation appeared to predate the DAPK signal. The activation of both AMPK and DAPK prompted the phosphorylation of p53 and enhanced Beclin 1 expression. Neither the downregulation of p38 MAPK by its siRNA or chemical inhibitor nor the upregulation of p38 MAPK by p38 MAPK DNA transfection affected B203580-induced autophagy. Collectively, the findings demonstrate a novel function of SB203580 to induce autophagy via activating AMPK and DAPK but independent of p38 MAPK. The induction of autophagy can thus account for the antiproliferative effect of SB203580 in HCC cells.[3] We report that SB203580 (SB), a specific inhibitor of p38-MAPK, protects pig myocardium against ischemic injury in an in vivo model. SB was applied by local infusion into the subsequently ischemic myocardium for 60 min before a 60-min period of coronary occlusion followed by 60-min reperfusion (index ischemia). Infarct size was reduced from a control value of 69.3 +/- 2.7% to 36.8 +/- 3.7%. When SB was infused systemically for 10 min before index ischemia, infarct size was reduced to 36.1 +/- 5.6%. We measured the content of phosphorylated p38-MAPK after systemic infusion of SB and Krebs-Henseleit buffer (KHB; negative control) and during the subsequent ischemic period using an antibody that reacts specifically with dual-phosphorylated p38-MAPK (Thr180/ Tyr182). Ischemia with and without SB significantly increased phospho-p38-MAPK, with a maximum reached at 20 min but was less at 30 and 45 min under the influence of the inhibitor. The systemic infusion of SB for 10 min before index ischemia did not significantly change the p38-MAPK activities (compared with vehicle, studied by in-gel phosphorylation) < or =20 min of ischemia, but activities were reduced at 30 and 45 min. Measurements of p38-MAPK activities in situations in which SB was present during in-gel phosphorylation showed significant inhibition of p38-MAPK activities. The systemic infusion of SB significantly inhibited the ischemia-induced phosphorylation of nuclear activating transcription factor 2 (ATF-2). Using a specific ATF-2 antibody, we did not observe significant changes in ATF-2 abundance when nuclear fractions from untreated, KHB-, and SB-treated tissues were compared. We investigated also the effect of local and systemic infusion of SB on the cardioprotection induced by ischemic preconditioning (IP). The infusions (local or systemic) of SB before and during the IP protocol did not influence the infarct size reduction mediated by IP. The observed protection of the myocardium against ischemic damage by SB points to the negative role of the p38-MAPK pathway during ischemia.[4] |
| Molecular Formula |
C21H16FN3OS
|
|---|---|
| Molecular Weight |
377.43
|
| Exact Mass |
377.099
|
| Elemental Analysis |
C, 66.83; H, 4.27; F, 5.03; N, 11.13; O, 4.24; S, 8.49
|
| CAS # |
152121-47-6
|
| Related CAS # |
Adezmapimod hydrochloride;869185-85-3
|
| PubChem CID |
176155
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Boiling Point |
615.6±55.0 °C at 760 mmHg
|
| Melting Point |
249 - 250ºC
|
| Flash Point |
326.1±31.5 °C
|
| Vapour Pressure |
0.0±1.7 mmHg at 25°C
|
| Index of Refraction |
1.715
|
| LogP |
4.1
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
4
|
| Heavy Atom Count |
27
|
| Complexity |
500
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S(C([H])([H])[H])(C1C([H])=C([H])C(=C([H])C=1[H])C1=NC(C2C([H])=C([H])C(=C([H])C=2[H])F)=C(C2C([H])=C([H])N=C([H])C=2[H])N1[H])=O
|
| InChi Key |
CDMGBJANTYXAIV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25)
|
| Chemical Name |
4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine;hydrochloride
|
| Synonyms |
RWJ 64809; PB 203580; Adezmapimod; 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole; 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine; 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine; RWJ64809; SB203580; SB203580; SB 203580; RWJ-64809; PB-203580; PB203580
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.62 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2 mg/mL (5.30 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. View More
Solubility in Formulation 3: 2 mg/mL (5.30 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. Solubility in Formulation 4: 2 mg/mL (5.30 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. Solubility in Formulation 5: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O: 5mg/mL Solubility in Formulation 6: 16.67 mg/mL (44.17 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6495 mL | 13.2475 mL | 26.4950 mL | |
| 5 mM | 0.5299 mL | 2.6495 mL | 5.2990 mL | |
| 10 mM | 0.2649 mL | 1.3247 mL | 2.6495 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
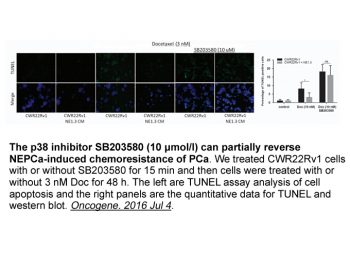 |
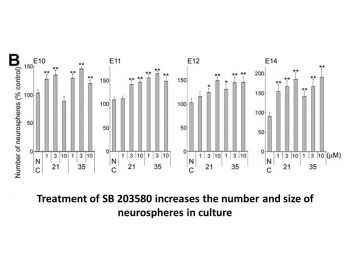 |