
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
SU11274 (also called PKI-SU11274; PKI-SU-11274) is a novel, potent and selective Met inhibitor with potential antineoplastic activity. It has no effect on PGDFRβ, EGFR, or Tie2, and in cell-free assays, it inhibits Met with an IC50 of 10 nM. SU11274 effectively inhibits the growth and migration of pancreatic cancer cells. Furthermore, it amplifies the DU145 prostate cancer cell line's reaction to ionizing radiation. With IC50 values ranging from 0.8 to 4.4 μM, SU11274 suppresses the phosphorylation of c-Met induced by hepatocyte growth factor and its subsequent signaling in c-Met-expressing non-small cell lung cancer (NSCLC) cells.
| Targets |
Met (IC50 = 10 nM)
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
The human c-Met cytoplasmic domain is fused to glutathione S-transferase (GST) to create a chimeric protein that is expressed in SF9 cells. An ELISA-based Met biochemical assay employing the random copolymer poly(Glu:Tyr) (4:1) immobilized on microtiter plates as a substrate makes use of the c-Met kinase GST-fusion protein. In a buffer containing 5 μM ATP, 10 mM MnCl2, 50 mM HEPES (pH 7.5), 25 mM NaCl, 0.01% BSA, and 0.1 mM Na orthovanadate, the IC50 value is calculated using different concentrations of SU11274. The kinase reaction is run at room temperature for five minutes. Using anti-pTyr antibodies conjugated with horseradish peroxidase, the degree of substrate phosphorylation is quantified.
|
||
| Cell Assay |
For 24, 48, and 72 hours, cells are exposed to different SU11274 concentrations with or without HGF. Trypan blue exclusion or the MTT assay are used to count the number of viable cells. Using propidium iodide staining and Annexin V-positive staining, respectively, fluorescence-activated cell sorter analysis measures the cell cycle and apoptosis.
|
||
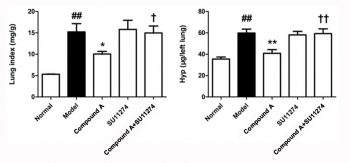
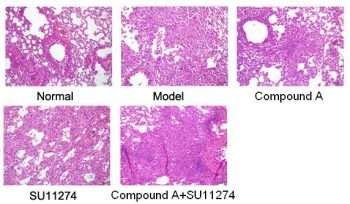
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
1h-indole-5-sulfonamide, n-(3-chlorophenyl)-3-[[3,5-dimethyl-4-[(4-methyl-1-piperazinyl)carbonyl]-1h-pyrrol-2-yl]methylene]-2,3-dihydro-n-methyl-2-oxo-, (3z)- is a sulfonamide.
|
| Molecular Formula |
C28H30CIN5O4S
|
|
|---|---|---|
| Molecular Weight |
568.09
|
|
| Exact Mass |
567.17
|
|
| Elemental Analysis |
C, 59.20; H, 5.32; Cl, 6.24; N, 12.33; O, 11.27; S, 5.64
|
|
| CAS # |
658084-23-2
|
|
| Related CAS # |
|
|
| PubChem CID |
9549297
|
|
| Appearance |
White to yellow solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Index of Refraction |
1.664
|
|
| LogP |
2.15
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
5
|
|
| Heavy Atom Count |
39
|
|
| Complexity |
1070
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
ClC1=C([H])C([H])=C([H])C(=C1[H])N(C([H])([H])[H])S(C1C([H])=C([H])C2=C(C=1[H])/C(/C(N2[H])=O)=C(\[H])/C1=C(C([H])([H])[H])C(=C(C([H])([H])[H])N1[H])C(N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)(=O)=O
|
|
| InChi Key |
FPYJSJDOHRDAMT-KQWNVCNZSA-N
|
|
| InChi Code |
InChI=1S/C28H30ClN5O4S/c1-17-25(30-18(2)26(17)28(36)34-12-10-32(3)11-13-34)16-23-22-15-21(8-9-24(22)31-27(23)35)39(37,38)33(4)20-7-5-6-19(29)14-20/h5-9,14-16,30H,10-13H2,1-4H3,(H,31,35)/b23-16-
|
|
| Chemical Name |
(3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylidene]-N-methyl-2-oxo-1H-indole-5-sulfonamide
|
|
| Synonyms |
SU-11274; PKI-SU11274; PKI SU11274; PKI-SU11274;SU 11274; SU11274
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.40 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 30% PEG400+0.5% Tween80+5% propylene glycol: 30 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7603 mL | 8.8014 mL | 17.6028 mL | |
| 5 mM | 0.3521 mL | 1.7603 mL | 3.5206 mL | |
| 10 mM | 0.1760 mL | 0.8801 mL | 1.7603 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
 |
 |