
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Tariquidar (formerly XR9576; D06008; XR-9576; D-06008) is a potent and selective noncompetitive inhibitor of P-glycoprotein (P-gp) with potential antineoplastic activity. It inhibits P-gp with a Kd of 5.1 nM in CHrB30 cell line, it reverses drug resistance in MDR cell Lines. Tariquidaris is currently undergoing research as an adjuvant against multidrug resistance in cancer. Tariquidar non-competitively binds to the p-glycoprotein transporter, thereby inhibiting transmembrane transport of anticancer drugs. Inhibition of transmembrane transport may result in increased intracellular concentrations of an anticancer drug, thereby augmenting its cytotoxicity.
| Targets |
P-gp (Kd = 5.1 nM)
|
||
|---|---|---|---|
| ln Vitro |
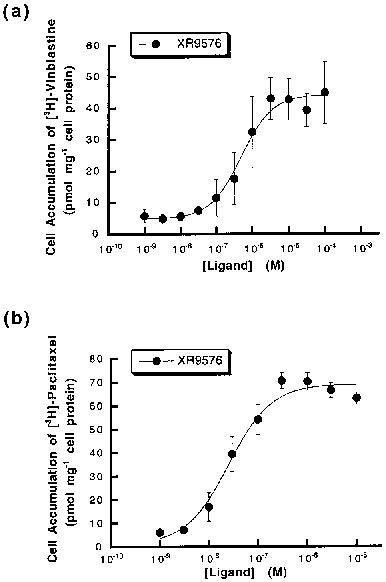
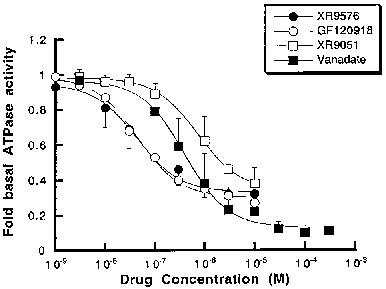
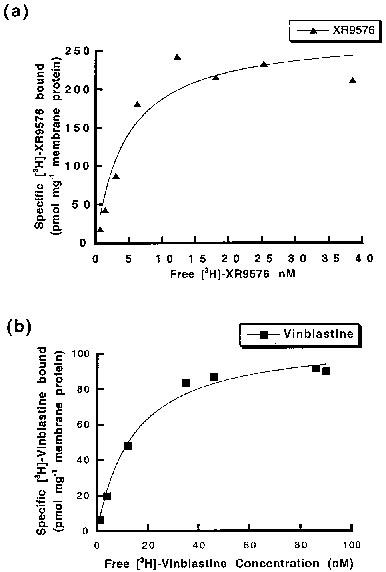
Tariquidar (XR9576) increases the steady-state accumulation of P-gp, making it a potent modulator of P-gp-mediated transport of [3H]-Vinblastine and [3H]-Paclitaxel. The levels of P-gp observed in AuxB1 cells (EC50=487±50 nM) are not required for the cytotoxic effects observed in CHrB30 cells. [3H]-Tariquidar has the strongest affinity (Kd=5.1±0.9 nM, n=7) and a binding capacity (Bmax) of 275±15 pmol/mg membrane protein when it comes to CHrB30 membranes. The modulator Tariquidar (EC50=487±50 nm) increased the accumulation of [3H]-vinblastine in a dose-dependent manner when compared to the parental cell line. With an effective IC50 value of 43±9 nM, the MDR modulator Tariquidar can inhibit 60–70% of vanadate-sensitive ATPase activity[1]. Several medications, such as doxorubicin, paclitaxel, etoposide, and vincristine, are made more cytotoxic by tiriquidar (XR9576); in the presence of 25–80 nM XR9576, resistance is completely reversed. Strong photoaffinity labeling of P-gp by [3H]Azidopine is inhibited by tariquidar, suggesting a direct interaction with the protein [2].
|
||
| ln Vivo |
In mice with intrinsically resistant MC26 colon cancers, coadministration of Tariquidar (XR9576) at a dose of 2.5–4.0 mg/kg maximally increased the anticancer efficacy of doxorubicin without appreciably increasing toxicity was noted. Moreover, in nude mouse xenografts of two highly resistant MDR human cancers (2780AD, H69/LX4), coadministration with Tariquidar (6–12 mg/kg po) completely restored the efficacy of paclitaxel, etoposide, and vincristine Antitumor activity. Additionally, when doxorubicin is subcutaneously injected in vivo against MC26 tumors, tartiquidar greatly increases its anti-tumor effectiveness [2].
|
||
| Enzyme Assay |
ATP hydrolytic activity of P-gp in CHrB30 membranes[1]
A previously described colorimetric assay was used to measure inorganic phosphate liberation following ATP hydrolysis (Chifflet et al., 1988). Membranes (1 μg protein) were incubated with Na2ATP (2 mm) in a total assay volume of 50 μl in buffer containing (mm): Tris pH 7.4 50, MgSO4 5, 0.02% NaN3, NH4Cl 150 for 20 min at 37°C. The ATPase activity was linear to 40 min at 37°C. Modulators (from DMSO stocks) and the ATPase inhibitor vanadate, were added in the concentration range 10−9–10–5 m. The final DMSO concentration was always <1%, a level known not to alter ATPase activity. The effect of drugs on the ATPase activity was fitted by the general dose-response relationship (see above). Specific drug binding to P-glycoprotein[1] A rapid filtration assay was used to measure the binding of [3H]-vinblastine, [3H]-paclitaxel and [3H]-XR9576 to P-gp in CHrB30 membranes as previously described (Ferry et al., 1992). Membranes were incubated with appropriate radioligand in a total buffer volume of 200 μl (50 mm Tris pH 7.4) for a period of 2–3 h to reach equilibrium. Washing buffer (3 ml) containing 20 mm MgSO4, 20 mm Tris (pH 7.4) was then added and the samples filtered under vacuum through a single GF/F filter in a filtration manifold to separate bound and free ligand. After further washing (2×3 ml) the amount of bound ligand was determined by liquid scintillation counting. Non-specific binding was defined as the amount of [3H]-ligand bound in the presence of at least a 100 fold excess of competing ligand (indicated in Results) and was subtracted from all values. Determination of the capacity and affinity of [3H]-ligand binding was achieved by saturation isotherm analysis. Membranes were incubated with increasing concentration of labelled drug and the amount bound (pmol mg−1) plotted as a function of free ligand concentration. |
||
| Cell Assay |
Cell culture[1]
The Chinese hamster ovary parental (sensitive) AuxB1 and the resistant CHrB30 cells were grown as previously described in α-minimum essential medium (α-MEM) containing 10% foetal calf serum (Kartner et al., 1983). The CHrB30 cells, derived from AuxB1 cells by step wise selection in colchicine (Kartner et al., 1983), express P-gp and selection pressure was maintained by supplementing media with 30 μg ml−1 colchicine. Plasma membrane preparation[1] Plasma membranes were prepared following disruption of CHrB30 cells using nitrogen cavitation and collection with sucrose density centrifugation as previously described (Lever, 1977). The final membrane preparation was stored at −70°C, at protein concentrations of 5–10 mg ml–1 in buffer containing 0.25 m sucrose, 10 mm Tris HCI (pH 7.5) and including the protease inhibitors leupeptin (0.1 mg ml−1), pepstatin A (0.1 mg ml−1) and benzamidine (1 mm). Steady-state drug accumulation assay[1] AuxB1 and CHrB30 cells were grown to confluency in 12-well (24 mm) tissue culture dishes and the steady-state accumulation of [3H]-vinblastine was measured as previously described (Martin et al., 1997). Accumulation was initiated by the addition of 0.1 μCi [3H]-vinblastine and unlabelled vinblastine to a final concentration of 100 nm. The accumulation of [3H]-paclitaxel was measured using 0.1 μCi [3H]-paclitaxel and unlabelled drug to a final concentration of 1 μm. Cells were incubated in a reaction volume of 1 ml for 60 min at 37°C under 5% CO2 in order to reach steady-state. The effect of the modulators XR9576 and GF120918 on [3H]-ligand accumulation was investigated in the concentration range 10−9–10−6 m. Modulators were added from a DMSO stock giving a final solvent concentration of 0.2% (v v−1). Following cell harvesting, accumulated drug was measured by liquid scintillation counting and normalized for cell protein content. Plots of amount accumulated as a function of modulator concentration were fitted with the general dose-response equation (De Lean et al., 1978). The accumulation of [3H]-XR9576 was also measured in AuxB1 and CHrB30 cells using several concentrations of radiolabelled drug (1–300 nm) in the presence and absence of 1 μm GF120918 and followed over a 60 min period, as described above. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
In contrast to human data, the present study found a high bioavailability of tariquidar in rats after oral dosing. Oral bioavailability was significantly higher (p = 0.032) for formulation B (86.3%) than for formulation A (71.6%). After intraperitoneal dosing bioavailability was 91.4% for formulation A and 99.6% for formulation B.
Conclusion: The present findings extend the available information on tariquidar and provide a basis for future studies involving oral administration of this compound.[5]
|
||
| References |
|
||
| Additional Infomation |
Tariquidar is a member of benzamides.
Tariquidar is an anthranilamide derivative with multidrug resistance properties. Tariquidar non-competitively binds to the p-glycoprotein transporter, thereby inhibiting transmembrane transport of anticancer drugs. Inhibition of transmembrane transport may result in increased intracellular concentrations of an anticancer drug, thereby augmenting its cytotoxicity. (NCI04) Drug Indication Investigated for use/treatment in ovarian cancer, lung cancer, and breast cancer. Mechanism of Action Tariquidar is an anthranilic acid derivative third generation P-glycoprotein (P-gp) inhibitors. P-gp is a transport protein found in normal cells that has many trasport and modulatory functions, from affecting the distribution and bioavailability of drugs to mediating the migration of dendritic cells. P-gp is responsible for multidrug resistance through transport of anti-cancer drugs out of their target cells. It is proposed that tariquidar's and its derivatives may bind to the H-binding site of P-gp through multiple mechanisms of binding. |
| Molecular Formula |
C38H38N4O6
|
|
|---|---|---|
| Molecular Weight |
646.73
|
|
| Exact Mass |
646.279
|
|
| Elemental Analysis |
C, 70.57; H, 5.92; N, 8.66; O, 14.84
|
|
| CAS # |
206873-63-4
|
|
| Related CAS # |
206873-63-4; 625375-84-0 (mesylate); 1992047-62-7 (2HCl); 625375-83-9 (methanesulfonate hydrate)
|
|
| PubChem CID |
148201
|
|
| Appearance |
Off-white to yellow solid
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
716.0±60.0 °C at 760 mmHg
|
|
| Flash Point |
386.8±32.9 °C
|
|
| Vapour Pressure |
0.0±2.3 mmHg at 25°C
|
|
| Index of Refraction |
1.662
|
|
| LogP |
6.38
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
11
|
|
| Heavy Atom Count |
48
|
|
| Complexity |
1040
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O(C([H])([H])[H])C1=C(C([H])=C2C(=C1[H])C([H])([H])N(C([H])([H])C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])N([H])C(C1=C([H])C(=C(C([H])=C1N([H])C(C1C([H])=NC3=C([H])C([H])=C([H])C([H])=C3C=1[H])=O)OC([H])([H])[H])OC([H])([H])[H])=O)C([H])([H])C2([H])[H])OC([H])([H])[H]
|
|
| InChi Key |
LGGHDPFKSSRQNS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C38H38N4O6/c1-45-33-18-25-14-16-42(23-28(25)19-34(33)46-2)15-13-24-9-11-29(12-10-24)40-38(44)30-20-35(47-3)36(48-4)21-32(30)41-37(43)27-17-26-7-5-6-8-31(26)39-22-27/h5-12,17-22H,13-16,23H2,1-4H3,(H,40,44)(H,41,43)
|
|
| Chemical Name |
N-[2-[[4-[2-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]carbamoyl]-4,5-dimethoxyphenyl]quinoline-3-carboxamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.87 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 30% propylene glycol, 5% Tween 80, 65% D5W: 30 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5462 mL | 7.7312 mL | 15.4624 mL | |
| 5 mM | 0.3092 mL | 1.5462 mL | 3.0925 mL | |
| 10 mM | 0.1546 mL | 0.7731 mL | 1.5462 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
 |
 |
|
|---|
|
|