
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
Purity: ≥98%
THZ1 (THZ-1) is a novel, potent, selective and covalent/irreversible CDK7 inhibitor (IC50 = 3.2 nM) with anticancer activity. It has exceptional ability to target a remote cysteine residue located outside of the canonical kinase domain, providing an unanticipated means of achieving selectivity for CDK7. THZ1 covalently modifies CDK7 by targeting C312 residue outside of the kinase domain, providing an unanticipated means of achieving covalent selectivity. THZ1 potently inhibits proliferation of Jurkat and Loucy T-ALL cell lines with IC50 values of 50nM and 0.55nM, respectively. In the kinase binding assay, THZ1 shows a good binding affinity with IC50 value of 3.2nM.
| Targets |
CDK7 (IC50 = 3.2 nM)
|
||
|---|---|---|---|
| ln Vitro |
Jurkat cells and Loucy cells are inhibited by THZ1, with IC50 values of 50 nM and 0.55 nM, respectively. CDK12 is inhibited by THZ1 (9, 27, 83, 250, 750, and 2500 nM), although at higher concentrations than CDK7. THZ1 (1 μM) phosphorylates CAK and RNAPII CTD irreversibly. In Hela S3 cells, THZ1 (2.5 μM) covalently targets a specific cysteine outside the CDK7 kinase domain to irreversibly prevent RNAPII CTD phosphorylation. In T-ALL cell lines, THZ1 (250 nM) causes a drop in anti-apoptotic proteins, most notably MCL-1 and XIAP, as well as an increase in the apoptotic index and lower cell proliferation [1]. With an IC50 ranging from 5 to 20 nM, all genotyped human (hSCLC) cell lines exhibit great sensitivity to THZ1 [3].
|
||
| ln Vivo |
THZ1 (10 mg/kg) exhibits potent killing of primary chronic lymphocytic leukemia (CLL) cells and antiproliferative activity on primary TALL cells and on human T-ALL xenografts in vivo [1]. THZ1 (10 mg/kg, iv) reduced tumor growth and demonstrated no toxicity in a human MYCN-amplified NB mouse model [4]. THZ1 (10 mg/kg, ip) fully suppresses esophageal squamous cell carcinoma tumor growth in animals without weight loss or other common adverse symptoms [5].
To compare the single agent potency of THZ1 with combination effect of drugs, we performed a combination study with THZ1 and PARP inhibitor Olaparib, a FDA-approved drug in relapsed ovarian cancer irrespective of BRCA1/2 status. We first conducted tolerability studies and found that THZ1 administered by intraperitoneal injection (IP) twice daily (BID) at 10 mg/kg was well-tolerated with no signs of overt toxicity as judged by body weight and animal behavior (data not shown). In efficacy studies, we first implanted ascites-derived ovarian tumor cells into the mice, and after 7 days assigned animals into four groups receiving vehicle control (10 ml/kg, PO, QD) or THZ1 (10 mg/kg, IP, BID) or Olaparib (100 mg/kg, PO, QD) or combo (THZ1 +Olaparib) for 27 days, with bioluminescent imaging performed at 5 timepoints (0, 6, 13, 20, and 27 days) (Figure 5A–B). Consistent with previous studies of THZ1 or Olaparib, mouse body weight was minimally affected by the inhibitor (Figure 5—figure supplement 1). In all the 11 independent PDX models investigated, the administration of THZ1 caused significant inhibition on tumor cell growth (Figure 5B–C). Notably, in four models (DF-149, 172, 83, and 86), THZ1 induced complete inhibition on tumor growth (Figure 4C, termed category i). In six models (DF-101, 106, 118, 20, 68, and 216), THZ1 first caused an obvious decrease of tumor burden but re-gained growth at later time points (termed category ii). Only one model (DF-181, termed category iii) did not demonstrate tumor regression and rather present slower tumor cell growth upon THZ1 treatment. The administration of Olaparib did not dramatically inhibit tumor growth, and only showed very modest effect in three models (DF-106, 68, and 83). The combination of THZ1 and Olaparib, however, displayed synergistic effect and further inhibition on tumor growth was observed in five models (DF-106, 118, 86, 181, and 68).In addition, we found that the protein abundance of both MYC and MCL-1 in the tumor was nearly abrogated following THZ1 treatment (Figure 5D). Overall, the potency of THZ1 in suppressing tumor growth in our ovarian tumor models is striking, given that tumor regression is rarely observed in previous studies using THZ1. The combination study indicated that combining THZ1 with clinical PARP inhibitors could be promising future therapeutic approach for treating ovarian cancer[2]. |
||
| Enzyme Assay |
Inhibitor treatment experiments [1]
Time-course experiments such as those described in Extended Data Fig. 5a were conducted to determine the minimal time required for full inactivation of CDK7. Cells were treated with THZ1, THZ1-R, or DMSO for 0–6 hrs to assess the effect of time on the THZ1 –mediated inhibition of RNAPII CTD phosphorylation. For subsequent experiments cells were treated with compounds for 4 hrs as determined by time-course experiment described above, unless otherwise noted. For inhibitor washout experiments (Fig. 2e, f; Extended Data Fig. 5) cells were treated with THZ1, THZ1-R, or DMSO for 4 hrs. Medium containing inhibitors was subsequently removed to effectively ‘washout’ the compound and the cells were allowed to grow in the absence of inhibitor. For each experiment, lysates were probed for RNAPII CTD phosphorylation and other specified proteins. |
||
| Cell Assay |
High-throughput cell line panel viability assay [1]
Cells were seeded in 384-well microplates at ~15% confluency in medium with 5% FBS and penicillin/streptavidin. Cells were treated with THZ1 or DMSO for 72 hrs and cell viability was determined using resazurin. Cell proliferation assays [2] After virus infection and selection with puromycin, cells were seeded in 12-well plates (at the density of 5 × 103) in 1 ml medium. 14 days later, cells were fixed with 1% formaldehyde for 15 minutes, and stained with crystal violet (0.05%, wt/vol), a chromatin-binding cytochemical stain for 15 minutes. The plates were washed extensively in plenty of deionized water, dried upsidedown on filter paper, and imaged with Epson scanner. For the 3-day cell proliferation assay in 96-well plate, cells were plated at the density of 6000 to 10,000 cells per well and treated with THZ1 or YKL-1–116 of various concentrations on the next day. After 72 hr incubation, CellTiter-Glo reagent was added to cells directly and luminescent signal was read on a plate reader |
||
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
THZ1 is a member of the class of indoles that is 1H-indole substituted by a 5-chloro-2-[3-(4-{[(2E)-4-(dimethylamino)but-2-enoyl]amino}benzamido)anilino]pyrimidin-4-yl group at position 3. It is a selective and potent covalent inhibitor of CDK7 that exhibits anti-proliferative effects in cancer cell lines. It has a role as an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor and an antineoplastic agent. It is a member of indoles, an aminopyrimidine, a member of benzamides, an organochlorine compound, an enamide and a secondary carboxamide.
Tumour oncogenes include transcription factors that co-opt the general transcriptional machinery to sustain the oncogenic state, but direct pharmacological inhibition of transcription factors has so far proven difficult. However, the transcriptional machinery contains various enzymatic cofactors that can be targeted for the development of new therapeutic candidates, including cyclin-dependent kinases (CDKs). Here we present the discovery and characterization of a covalent CDK7 inhibitor, THZ1, which has the unprecedented ability to target a remote cysteine residue located outside of the canonical kinase domain, providing an unanticipated means of achieving selectivity for CDK7. Cancer cell-line profiling indicates that a subset of cancer cell lines, including human T-cell acute lymphoblastic leukaemia (T-ALL), have exceptional sensitivity to THZ1. Genome-wide analysis in Jurkat T-ALL cells shows that THZ1 disproportionally affects transcription of RUNX1 and suggests that sensitivity to THZ1 may be due to vulnerability conferred by the RUNX1 super-enhancer and the key role of RUNX1 in the core transcriptional regulatory circuitry of these tumour cells. Pharmacological modulation of CDK7 kinase activity may thus provide an approach to identify and treat tumour types that are dependent on transcription for maintenance of the oncogenic state.[1] High-grade serous ovarian cancer is characterized by extensive copy number alterations, among which the amplification of MYC oncogene occurs in nearly half of tumors. We demonstrate that ovarian cancer cells highly depend on MYC for maintaining their oncogenic growth, indicating MYC as a therapeutic target for this difficult-to-treat malignancy. However, targeting MYC directly has proven difficult. We screen small molecules targeting transcriptional and epigenetic regulation, and find that THZ1 - a chemical inhibiting CDK7, CDK12, and CDK13 - markedly downregulates MYC. Notably, abolishing MYC expression cannot be achieved by targeting CDK7 alone, but requires the combined inhibition of CDK7, CDK12, and CDK13. In 11 patient-derived xenografts models derived from heavily pre-treated ovarian cancer patients, administration of THZ1 induces significant tumor growth inhibition with concurrent abrogation of MYC expression. Our study indicates that targeting these transcriptional CDKs with agents such as THZ1 may be an effective approach for MYC-dependent ovarian malignancies.[2] Small cell lung cancer (SCLC) is an aggressive disease with high mortality, and the identification of effective pharmacological strategies to target SCLC biology represents an urgent need. Using a high-throughput cellular screen of a diverse chemical library, we observe that SCLC is sensitive to transcription-targeting drugs, in particular to THZ1, a recently identified covalent inhibitor of cyclin-dependent kinase 7. We find that expression of super-enhancer-associated transcription factor genes, including MYC family proto-oncogenes and neuroendocrine lineage-specific factors, is highly vulnerability to THZ1 treatment. We propose that downregulation of these transcription factors contributes, in part, to SCLC sensitivity to transcriptional inhibitors and that THZ1 represents a prototype drug for tailored SCLC therapy.[3] The MYC oncoproteins are thought to stimulate tumor cell growth and proliferation through amplification of gene transcription, a mechanism that has thwarted most efforts to inhibit MYC function as potential cancer therapy. Using a covalent inhibitor of cyclin-dependent kinase 7 (CDK7) to disrupt the transcription of amplified MYCN in neuroblastoma cells, we demonstrate downregulation of the oncoprotein with consequent massive suppression of MYCN-driven global transcriptional amplification. This response translated to significant tumor regression in a mouse model of high-risk neuroblastoma, without the introduction of systemic toxicity. The striking treatment selectivity of MYCN-overexpressing cells correlated with preferential downregulation of super-enhancer-associated genes, including MYCN and other known oncogenic drivers in neuroblastoma. These results indicate that CDK7 inhibition, by selectively targeting the mechanisms that promote global transcriptional amplification in tumor cells, may be useful therapy for cancers that are driven by MYC family oncoproteins. Objectives: Oesophageal squamous cell carcinoma (OSCC) is an aggressive malignancy and the major histological subtype of oesophageal cancer. Although recent large-scale genomic analysis has improved the description of the genetic abnormalities of OSCC, few targetable genomic lesions have been identified, and no molecular therapy is available. This study aims to identify druggable candidates in this tumour. Design: High-throughput small-molecule inhibitor screening was performed to identify potent anti-OSCC compounds. Whole-transcriptome sequencing (RNA-Seq) and chromatin immunoprecipitation sequencing (ChIP-Seq) were conducted to decipher the mechanisms of action of CDK7 inhibition in OSCC. A variety of in vitro and in vivo cellular assays were performed to determine the effects of candidate genes on OSCC malignant phenotypes. [4] Results: The unbiased high-throughput small-molecule inhibitor screening led us to discover a highly potent anti-OSCC compound, THZ1, a specific CDK7 inhibitor. RNA-Seq revealed that low-dose THZ1 treatment caused selective inhibition of a number of oncogenic transcripts. Notably, further characterisation of the genomic features of these THZ1-sensitive transcripts demonstrated that they were frequently associated with super-enhancer (SE). Moreover, SE analysis alone uncovered many OSCC lineage-specific master regulators. Finally, integrative analysis of both THZ1-sensitive and SE-associated transcripts identified a number of novel OSCC oncogenes, including PAK4, RUNX1, DNAJB1, SREBF2 and YAP1, with PAK4 being a potential druggable kinase. Conclusions: Our integrative approaches led to a catalogue of SE-associated master regulators and oncogenic transcripts, which may significantly promote both the understanding of OSCC biology and the development of more innovative therapies.[5] |
| Molecular Formula |
C31H28CLN7O2
|
|
|---|---|---|
| Molecular Weight |
566.05
|
|
| Exact Mass |
565.199
|
|
| Elemental Analysis |
C, 65.78; H, 4.99; Cl, 6.26; N, 17.32; O, 5.65
|
|
| CAS # |
1604810-83-4
|
|
| Related CAS # |
bio-THZ1;1604811-14-4;THZ1-R;1621523-07-6;THZ1 Hydrochloride
|
|
| PubChem CID |
73602827
|
|
| Appearance |
Off-white to yellow solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Index of Refraction |
1.735
|
|
| LogP |
5.08
|
|
| Hydrogen Bond Donor Count |
4
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
9
|
|
| Heavy Atom Count |
41
|
|
| Complexity |
896
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
CN(C)C/C=C/C(=O)NC1=CC=C(C=C1)C(=O)NC2=CC=CC(=C2)NC3=NC=C(C(=N3)C4=CNC5=CC=CC=C54)Cl
|
|
| InChi Key |
OBJNFLYHUXWUPF-IZZDOVSWSA-N
|
|
| InChi Code |
InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+
|
|
| Chemical Name |
N-[3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]phenyl]-4-[[(E)-4-(dimethylamino)but-2-enoyl]amino]benzamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 5 mg/mL (8.83 mM) in 10% DMSO + 90% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.42 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.42 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 2.08 mg/mL (3.67 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7666 mL | 8.8331 mL | 17.6663 mL | |
| 5 mM | 0.3533 mL | 1.7666 mL | 3.5333 mL | |
| 10 mM | 0.1767 mL | 0.8833 mL | 1.7666 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
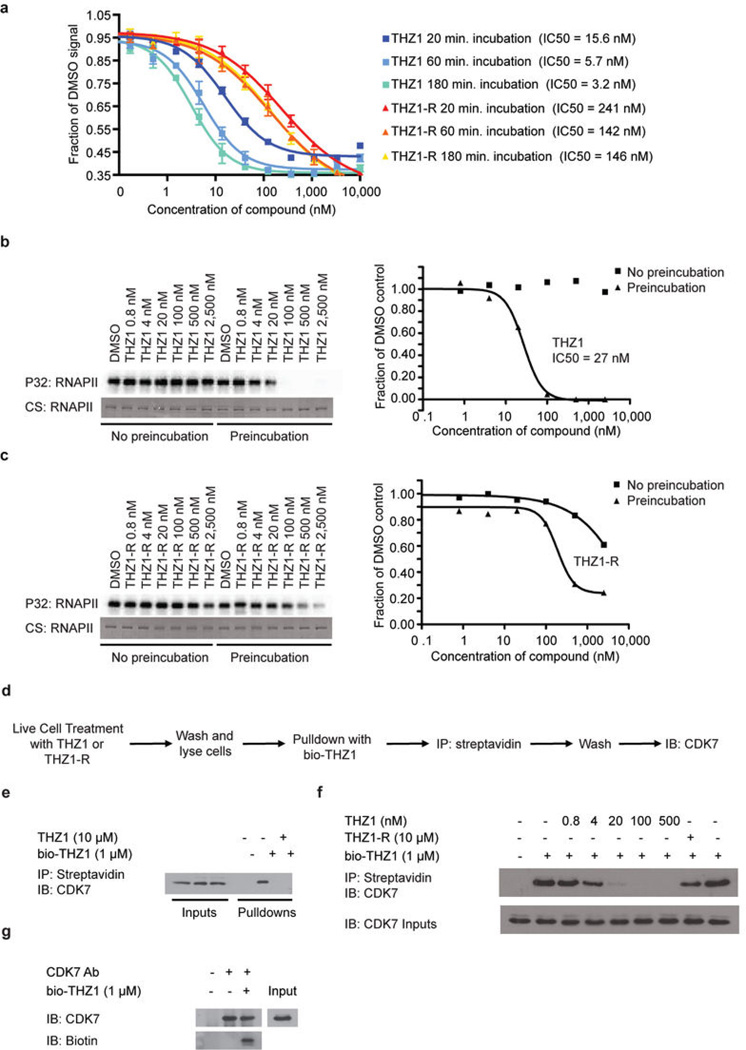 .THZ1 demonstrates time-dependent inhibition of CDK7in vitroand covalent binding of intracellular CDK7.Nature.2014 Jul 31;511(7511):616-20. .THZ1 demonstrates time-dependent inhibition of CDK7in vitroand covalent binding of intracellular CDK7.Nature.2014 Jul 31;511(7511):616-20. |
|---|
THZ1 covalently binds CDK7 C312.Nature.2014 Jul 31;511(7511):616-20. |
THZ1 inhibits CDK12 but at higher concentrations compared to CDK7.Nature.2014 Jul 31;511(7511):616-20. |