
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Tofacitinib citrate (formerly CP-690550 citrate), the citrate salt of tofacitinib (tasocitinib or CP 690550, brand Xeljanz), is a novel, potent and orally bioavailable inhibitor of JAK3 (Janus-Associated kinase) with IC50 of 1 nM in cell-free assays. In May 2018, the U.S. FDA approved tofacitinib citrate for the treatment of adult patients with moderately to severely active ulcerative colitis. It is the first oral drug approved for chronic use in ulcerative colitis. The inhibition is JAK3 specific with a selectivity 1000-fold more than other non-JAK family kinases. Besides inhibiting JAK3 (with IC50 of 1 nM), tofacitinib also inhibits JAK2 and JAK1 with 20- and 100-fold less in potency respectively. However, in a recent study, the binding affinities (Ki) of tofacitinib towards JAK1, JAK2, and JAK3 were reported to be 1.6 nM, 21.7 nM, and 6.5 nM respectively.
| Targets |
JAK3 (IC50 = 1 nM); JAK2 (IC50 = 20 nM); JAK1 (IC50 = 112 nM); Rock-II (IC50 = 3400 nM); Lck (IC50 = 3870 nM)
|
||
|---|---|---|---|
| ln Vitro |
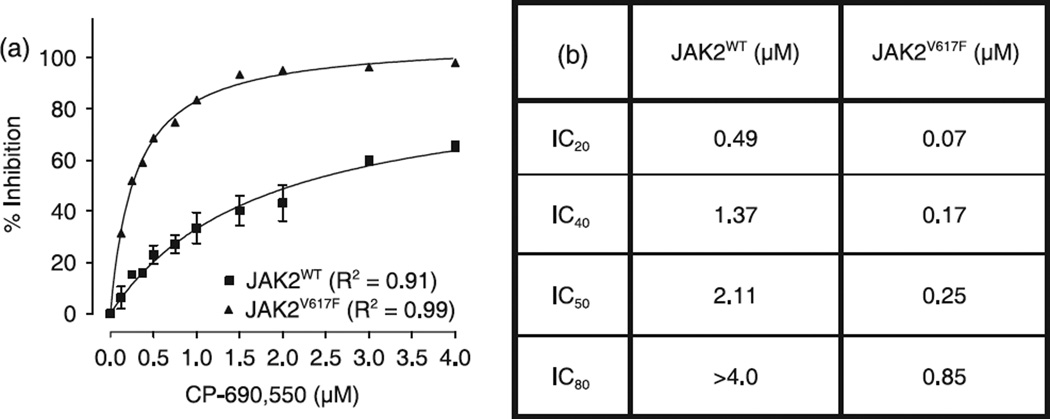
At 2.2 nM and 5 nM (Kd), tofacitinib (CP-690550) citrate binds possibly at JAK3 and JAK2. Additional binding for tofacitinib is reported for the following sites: Camk1 (Kd of 5,000 nM), DCamkL3 (Kd of 4.5 nM), Mst2 (Kd of 4,300 nM), Pkn1 (Kd of 200 nM), Rps6ka2 (Kin.Dom.2-C-terminal) (Kd of 1,400 nM), Rps6ka6 (Kin.Dom.2-C-terminal) (Kd of 1,200 nM), Snark (Kd of 420 nM), Tnk1 (Kd of 640 nM), and Tyk2 (Kd of 620 nM)[1]. To measure tyrosine kinase inhibitor (TKI) activity, K562, KCL22, and THP-1 cells are treated to varying dosages of STI571 or JAK inhibitors for a duration of 72 hours. The MTT assay is then used to assess the suppression of cell growth. IMA inhibits K562 and KCL22 cell proliferation in a concentration-dependent manner, but not THP-1 cell proliferation. IMA's IC50 values for K562 and KCL22 are 0.28 μM and 0.17 μM, respectively. Tofacitinib (TOF) and INCB018424 together increase the sensitivity of K562 and KCL22 to IMA, even if they do not decrease cell growth on their own[4].
|
||
| ln Vivo |
When compared to PEG-treated control mice, animals treated with tofacitinib exhibit a markedly reduced generation of anti-drug antibodies (ADAs) (during five weeks following initial immunization, p<0.01, n=8). Furthermore, day 28 is when ADAs are first noticeable. From days 21 through 35, there is a noticeable difference in titers to SS1P of 1000 to 200 times, respectively. Mice injected with keyhole limpet hemocyanin (KLH) produce antibodies more quickly than those given SS1P. However, when compared to controls, the treatment of tofacitinib lowers anti-KLH titers (p<0.05 on day 21 and p<0.01 on day 28, respectively, n = 5). Between days 21 and 28, there were reductions in titers ranging from 5000 to 250 fold, respectively[2]. A daily dose of tofacitinib of 6.2 mg/kg is chosen based on prior dose-response studies in order to produce 80% inhibition of hind paw volume and plasma exposure, which can suppress the JAK1 and JAK3 signaling pathways for more than 4 hours[3].
For five weeks following their initial immunization (p<0.01, n=8), animals treated with tofacitinib exhibit a considerably decreased development of anti-drug antibodies (ADAs) when compared to PEG-treated control mice. Additionally, day 28 is when ADAs become noticeable. Titers to SS1P show a 1000- to 200-fold variation from days 21 to 35, respectively. Keyhole limpet hemocyanin (KLH)-injected animals produce an antibody response more quickly than those treated with SS1P. Nevertheless, tofacitinib dosing lowers anti-KLH titers in comparison to controls (p<0.05 on day 21 and p<0.01 on day 28, respectively, n = 5). From days 21 through 28, titer reductions varied from 5000 to 250 fold[2]. The JAK1 and JAK3 signaling pathways can be suppressed for more than 4 hours with a daily dose of tofacitinib of 6.2 mg/kg, which is chosen based on prior dose-response experiments and provides 80% inhibition of hind paw volume and plasma exposure[3]. Tofacitinib by oral route inhibited the LPS-induced airway neutrophilia, the levels of some cytokines in the BALF and the phosphorylation of STAT3 in the lung tissue. Conclusions and implications: In summary, this study shows that JAK inhibition ameliorates inhaled LPS-induced airway inflammation in rats, suggesting that at least JAK/STAT3 signalling is involved in the establishment of the pulmonary neutrophilia induced by LPS. JAKs inhibitors should be further investigated as a potential therapy for respiratory inflammatory diseases.[4] Immunogenicity remains the "Achilles' heel" of protein-based therapeutics. Anti-drug Abs produced in response to protein therapeutics can severely limit both the safety and efficacy of this expanding class of agent. In this article, we report that monotherapy of mice with tofacitinib (the JAK inhibitor) quells Ab responses to an immunotoxin derived from the bacterial protein Pseudomonas exotoxin A, as well as to the model Ag keyhole limpet hemocyanin. Thousand-fold reductions in IgG1 titers to both Ags were observed 21 d post immunization. In fact, suppression was evident for all IgG isotypes and IgM. A reduction in IgG3 production was also noted with a thymus-independent type II Ag. Mechanistic investigations revealed that tofacitinib treatment led to reduced numbers of CD127+ pro-B cells. Furthermore, we observed fewer germinal center B cells and the impaired formation of germinal centers of mice treated with tofacitinib. Because normal Ig levels were still present during tofacitinib treatment, this agent specifically reduced anti-drug Abs, thus preserving the potential efficacy of biological therapeutics, including those used as cancer therapeutics[2]. |
||
| Enzyme Assay |
Kinase profiles were performed by a CRO utilizing KINOMEscan™. Activity is recorded via a competition binding assay of selected kinases that are fused to a proprietary tag. Measurements of the amount of kinase bound to an immobilized, active-site directed ligand in the presence and absence of the test compound provide a % of DMSO control for binding of ligand. Activities between 0 and 10 were selected for Kd determinations. Dendrogram representations were generated by an in-house visualization tool designated PhyloChem. Dendrogram clustering and apexes are based on the human phylogenetic kinase data available at http://kinase.com/human/kinome[1].
|
||
| Cell Assay |
Characterization of B cell differentiation and proliferation[2]
Splenocyte and bone marrow cell suspensions were prepared from BALB/c mice and total cells were counted. Cells (1×106) were stained with various combinations of the following anti-murine antibodies): CD3, B220, CD43, IgM, Fas, GL-7, CD24, BP-1, CD127 or IgG1-conjugated with FITC, PE or APC and analyzed on a FACSCalibur flow cytometer. At least 10,000 live events were acquired. For assessment of in vitro B cell proliferation CD43- splenocytes were MACS purified and labeled with 1 μM CFSE according to the manufacturer’s instructions. Labeled cells were activated for 48 hours with 25 μg/mL LPS (Escherichia coli 0111:B4) and 5 ng/ml IL-4 in the presence of 0, 0.1, 0.3, or 1.0 μM tofacitinib. Following culture, cells were washed, surface stained and examined by flow cytometry. In vitro human osteoclast differentiation and function.[3] Primary human monocytes were obtained by negative selection of CD14+ cells from leukopaks using magnetic-activated cell sorting (MACS) cell separation technology. Cells were plated in 96-well black tissue culture plates at 1 × 105 cells/well in high-glucose Dulbecco's modified Eagle's medium containing 5% fetal bovine serum (FBS) and 10 units/ml penicillin–streptomycin. Cultured cells were treated every other day for 14 days with either 25 ng/ml recombinant human macrophage colony-stimulating factor (M-CSF) for macrophage differentiation, or M-CSF in the presence of 100 ng/ml of recombinant human RANKL for osteoclast differentiation. Cells were also treated with or without varying concentrations of tofacitinib in 0.2% DMSO at the same time as differentiating cytokines. TRAP activity was quantified using ELF-97 fluorescent phosphatase substrate. Cells were then fixed and stained using a Leukocyte Acid Phosphatase kit according to the recommendations of the manufacturer.[3] Functional bone resorptive activity of human osteoclasts was measured by OsteoLyse assay. Human osteoclast precursor cells (Lonza) were plated at 1 × 104 cells/well in medium containing 33 ng/ml M-CSF and 66 ng/ml RANKL on 96-well OsteoLyse cell culture plates precoated with europium-conjugated human type I collagen. During the differentiation phase (days 0–6), cells were treated with varying concentrations of tofacitinib or left untreated. After 6 days in culture, fresh medium containing M-CSF and RANKL was added, and tofacitinib was replaced at the same concentrations or added to previously untreated cells. Additionally, alendronate sodium was added to untreated cells as a positive control. Cells were cultured for an additional 4 days to allow collagen release by functionally active osteoclasts, and culture supernatants were assayed for europium fluorescence using OsteoLyse Fluorophore-Releasing Reagent with measurement of time-resolved fluorescence over a 400 μsec interval at 340 nm excitation and 615 nm emission. In vitro human T lymphocyte RANKL production.[3] CD4+ T lymphocytes were negatively selected from a leukopak using MACS cell separation technology and cultured at 2.5 × 105 cells/well in round-bottomed 96-well tissue culture plates in RPMI 1640 medium containing glucose, 10% FBS, and 10 units/ml penicillin–streptomycin. Cells were treated with or without varying concentrations of tofacitinib in 0.2% DMSO and activated for 5 days with 1 μg/ml anti-human CD3 and 0.1 μg/ml anti-human CD28 antibodies together with 50 ng/ml recombinant human IL-2. RANKL secreted into culture medium was measured using a human LincoPlex assay. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Absorption 74% oral absorption (absolute bioavailability), with peak plasma concentrations (T max) achieved in 0.5-1 hour. Administration with fatty meals does not alter AUC but reduces Cmax by 32%. Route of Elimination 70% metabolized in the liver by CYP3A4 (major) and CYP2C19 (minor). Metabolites produced are inactive. 30% renally eliminated as unchanged drug. Volume of Distribution Vd= 87L after intravenous administration. Distribution is equal between red blood cells and plasma. The protein binding of tofacitinib is approximately 40%. Tofacitinib binds predominantly to albumin and does not appear to bind to a1-acid glycoprotein. Tofacitinib distributes equally between red blood cells and plasma. The absolute oral bioavailability of tofacitinib is 74%. Coadministration of Xeljanz with a high-fat meal resulted in no changes in AUC while Cmax was reduced by 32%. In clinical trials, Xeljanz was administered without regard to meals. Clearance mechanisms for tofacitinib are approximately 70% hepatic metabolism and 30% renal excretion of the parent drug. The metabolism of tofacitinib is primarily mediated by CYP3A4 with minor contribution from CYP2C19. In a human radiolabeled study, more than 65% of the total circulating radioactivity was accounted for by unchanged tofacitinib, with the remaining 35% attributed to 8 metabolites, each accounting for less than 8% of total radioactivity. The pharmacologic activity of tofacitinib is attributed to the parent molecule. Following oral administration of Xeljanz /to humans/, peak plasma concentrations are reached within 0.5-1 hour, elimination half-life is approximately 3 hours and a dose-proportional increase in systemic exposure was observed in the therapeutic dose range. Steady state concentrations are achieved in 24-48 hours with negligible accumulation after twice daily administration. /MILK/ Tofacitinib was secreted in milk of lactating rats. It is not known whether tofacitinib is excreted in human milk. View More
Metabolism / Metabolites
Biological Half-Life: ~3 hours The elimination half-life of tofacitinib /in humans/ is approximately 3 hours. Protein Binding: 40%, mostly bound to albumin. |
||
| Toxicity/Toxicokinetics |
Toxicity Summary
IDENTIFICATION AND USE: Tofacitinib is a yellow foam. As the drug Xeljanz, it is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to methotrexate. It may be used as monotherapy or in combination with methotrexate or other nonbiologic disease-modifying antirheumatic drugs (DMARDs). HUMAN EXPOSURE AND TOXICITY: According to epidemiological studies, the overall risk of infection (including serious infection) and mortality rates in RA patients treated with tofacitinib appear to be similar to those observed in RA patients treated with biologic agents. The rates of serious infection were stable over time. Within the global tofacitinib RA development program, tuberculosis was the most common opportunistic infection reported but was rare in regions of low and medium TB incidence. In a genotoxicity study, increases in chromosomal abnormalities were observed in a human lymphocyte in vitro cytogenetic assay, at high cytotoxic concentrations with metabolic activation, but no effects were observed without metabolic activation. ANIMAL STUDIES: In cynomolgus monkeys, emesis and decreased activity were observed as a result of acute exposure. Xeljanz caused death in rats at single oral doses of >/= 500 mg/kg. Immune and hematopoietic organ systems were identified as main targets in repeat-dose toxicity studies on animals. In a peri/postnatal development study in rats, Xeljanz decreased the number of delivered and live born pups, and reduced pup survival at oral doses of 50 mg/kg/day. Xeljanz was teratogenic (external, visceral and skeletal abnormalities) in rabbits and rats at oral doses of 30 and 100 mg/kg/day, respectively. Xeljanz was not mutagenic in the bacterial reverse mutation assay. Xeljanz was not mutagenic in mammalian cells (in vitro CHO/HGPRT assay) and did not induce primary DNA damage in an in vivo/in vitro rat hepatocyte unscheduled DNA synthesis assay. Xeljanz was also negative in the in vivo rat micronucleus test. In a 2-year rat carcinogenicity study, Xeljanz induced benign Leydig cell tumors and malignant hibernomas at oral doses of >/= 30 mg/kg/day and benign thymomas at 100/75 mg/kg/day. In a 39-week repeat-dose toxicity study in adult monkeys, lymphomas were observed at the high dose of 5 mg/kg twice daily, but not at the lower dose of 1 mg/kg twice daily (approximately equivalent to human exposure). Hepatotoxicity In large registration clinical trials, serum aminotransferase elevations occurred in 28% to 34% of tofacitinib treated subjects compared to 25% in comparator arms and 10% in placebo recipients. These elevations were typically mild and transient, but values above 3 times the upper limit of normal (ULN) occurred in 1% to 2% of patients on tofacitinib compared to less than 1% on placebo. The elevations occasionally led to early discontinuations, but more often resolved even without dose adjustment. In prelicensure studies, there were no instances of clinically apparent liver injury attributed to tofacitinib. Since approval and more wide scale availability of tofacitinib, there have been no published reports of hepatotoxicity associated with its use but a proportion of patients do develop serum aminotransferase elevations which in some cases leads to drug discontinuation. While other Janus kinase inhibitors such as ruxolitinib have been associated with episodes of reactivation of hepatitis B, spontaneous reports of clinically apparent reactivation of hepatitis during tofacitinib therapy have not been reported. On the other hand, retrospective studies on patients with HBsAg and inactive liver disease who were treated with tofacitinib have been reported to develop rising levels of HBV DNA and modest elevations in serum aminotransferase levels without symptoms. In contrast, studies of patients with anti-HBc without HBsAg in serum have shown no evidence of HBV DNA rises and appearance of HBsAg. Thus, reactivation of hepatitis B during therapy can occur, although it is generally mild and self-limited in course. Whether reactivation of hepatitis B can arise after therapy of susceptible patients with tofacitinib for severe COVID-19 pneumonia is unknown, but there have been no such reports to date. Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury with the potential to cause reactivation of hepatitis B). View More
Effects During Pregnancy and Lactation
◈ What is tofacitinib? Tofacitinib is a medication that has been used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. You can find more information on these conditions in the MotherToBaby fact sheets at https://mothertobaby.org/fact-sheets/rheumatoid-arthritis/, https://mothertobaby.org/fact-sheets/psoriasis-and-pregnancy/ and https://mothertobaby.org/fact-sheets/inflammatory-bowel-disease-pregnancy/. A brand name of tofacitinib is Xeljanz® and Xeljanz XR®.Tofacitinib is also being studied to treat severe COVID-19. Since there is little information about tofacitinib in pregnancy and breastfeeding, it is not currently recommended for treatment of COVID-19 in people who are pregnant or breastfeeding if other recommended treatment options are available. However, the National Institutes of Health (NIH) also state that necessary COVID-19 treatments should not be withheld from people just because they are pregnant or breastfeeding. More information on COVID-19 can be found in our fact sheet here: https://mothertobaby.org/fact-sheets/covid-19/.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take tofacitinib. Can it make it harder for me to get pregnant? It is not known if tofacitinib can make it harder to get pregnant. ◈ Does taking tofacitinib increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done to see if tofacitinib increases the chance of miscarriage. Reports of people exposed to tofacitinib during early pregnancy have not suggested an increased chance of miscarriage. ◈ Does taking tofacitinib increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. It is not known if tofacitinib increases the chance of birth defects above the background risk. Animal studies showed an increase in birth defects with the use of tofacitinib at much higher doses than those used in humans. No increased chance of birth defects has been reported in cases of people exposed to tofacitinib during early pregnancy. ◈ Does taking tofacitinib in pregnancy increase the chance of other pregnancy-related problems? Studies have not been done to see if tofacitinib increases the chance of pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). ◈ Does taking tofacitinib in pregnancy affect future behavior or learning for the child? Studies have not been done to see if tofacitinib can cause behavior or learning issues for the child. ◈ Breastfeeding while taking tofacitinib: Tofacitinib has not been well studied for use during breastfeeding. The manufacturer and an expert panel recommend that breastfeeding be stopped while using tofacitinib and for 18 hours after the last dose. For the extended release form (Xeljanz® XR), they recommend waiting 36 hours after the last dose before breastfeeding again. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes tofacitinib, could it affect fertility or increase the chance of birth defects? Studies have not been done to see if tofacitinib could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Interactions In healthy individuals, the CYP3A inducer rifampin (600 mg orally once daily for 7 days) decreased peak plasma concentrations and AUC of tofacitinib (single oral dose of 30 mg) by 74 and 84%, respectively. Concomitant use of rifampin may decrease efficacy of tofacitinib. There is a risk of added immunosuppression when Xeljanz is coadministered with potent immunosuppressive drugs (e.g., azathioprine, tacrolimus, cyclosporine). Combined use of multiple-dose Xeljanz with potent immunosuppressants has not been studied in rheumatoid arthritis. Use of Xeljanz in combination with biologic disease-modifying antirheumatic drugs (DMARDS) or potent immunosuppressants such as azathioprine and cyclosporine is not recommended. Concomitant use of tofacitinib with potent immunosuppressive agents (e.g., azathioprine, cyclosporine, tacrolimus) increases the risk of immunosuppression and is not recommended. Concomitant use of tofacitinib with such agents in patients with rheumatoid arthritis has not been studied to date. In healthy individuals, cyclosporine (200 mg orally every 12 hours for 5 days) decreased the clearance of tofacitinib (single oral dose of 10 mg), resulting in a 73% increase in the AUC of tofacitinib, accompanied by a 17% decrease in peak plasma tofacitinib concentrations. In healthy individuals, tacrolimus (5 mg orally every 12 hours for 7 days) slightly decreased the clearance of tofacitinib (single oral dose of 10 mg), resulting in a 21% increase in the AUC of tofacitinib, accompanied by a 9% decrease in peak plasma tofacitinib concentrations. Tofacitinib exposure is decreased when Xeljanz is coadministered with potent CYP3A4 inducers (e.g., rifampin). |
||
| References |
|
||
| Additional Infomation |
Tofacitinib citrate is a citrate salt obtained by combining equimolar amounts of tofacitinib and citric acid. Used to treat moderately to severely active Rheumatoid Arthritis. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor and an antirheumatic drug. It contains a tofacitinib.
Tofacitinib Citrate is the citrate salt form of tofacitinib, an orally bioavailable inhibitor of Janus kinases (JAK), with immunomodulatory and anti-inflammatory activities. Upon oral administration, tofacitinib binds to JAK and prevents the activation of the JAK-signal transducers and activators of transcription (STAT) signaling pathway. This may decrease the production of pro-inflammatory cytokines, such as interleukin (IL)-6, -7, -15, -21, interferon-alpha (IFN-a) and -beta (IFN-b), and may prevent both an inflammatory response and the inflammation-induced damage caused by certain immunological diseases. JAK kinases are intracellular enzymes involved in signaling pathways affecting hematopoiesis, immunity and inflammation. See also: Tofacitinib (has active moiety). |
| Molecular Formula |
C22H28N6O8
|
|---|---|
| Molecular Weight |
504.4931
|
| Exact Mass |
504.196
|
| Elemental Analysis |
C, 52.38; H, 5.59; N, 16.66; O, 25.37
|
| CAS # |
540737-29-9
|
| Related CAS # |
Tofacitinib;477600-75-2;Tofacitinib-d3 citrate;2701680-77-3;(3S,4S)-Tofacitinib;1092578-47-6;(3R,4S)-Tofacitinib;1092578-46-5;(3S,4R)-Tofacitinib;1092578-48-7
|
| PubChem CID |
10174505
|
| Appearance |
White to off-white solid powder
|
| LogP |
0.234
|
| Hydrogen Bond Donor Count |
5
|
| Hydrogen Bond Acceptor Count |
12
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
36
|
| Complexity |
716
|
| Defined Atom Stereocenter Count |
2
|
| SMILES |
C[C@@H]1CCN(C[C@@H]1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N.C(C(=O)O)C(CC(=O)O)(C(=O)O)O
|
| InChi Key |
SYIKUFDOYJFGBQ-YLAFAASESA-N
|
| InChi Code |
InChI=1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
|
| Chemical Name |
2-hydroxypropane-1,2,3-tricarboxylic acid;3-[(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile
|
| Synonyms |
CP-690550; CP690550; CP 690550; Tasocitinib; Tofacitinib; Xeljanz (Trade name); CP-690550-10; Tofacitinib citrate; 540737-29-9; Tasocitinib citrate; Xeljanz; CP-690550 citrate; Tofacitinib (CP-690550) Citrate; Tofacitinib (citrate); Xeljanz Xr; CP-690,550-10; CP-690550 citrate;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: 100 mg/mL (198.2 mM)
Water:<1 mg/mL
Ethanol:<1 mg/mL
|
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.96 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.96 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.96 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: ≥ 1.43 mg/mL (2.83 mM) (saturation unknown) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 5: 0.5% methylcellulose:30mg/mL Solubility in Formulation 6: 2.5 mg/mL (4.96 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9822 mL | 9.9110 mL | 19.8220 mL | |
| 5 mM | 0.3964 mL | 1.9822 mL | 3.9644 mL | |
| 10 mM | 0.1982 mL | 0.9911 mL | 1.9822 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06202560 | Enrolling by invitation | Drug: Tofacitinib 5 MG | Frontal Fibrosing Alopecia Lichen Planopilaris |
Institute of Dermatology, Thailand | November 29, 2023 | Not Applicable |
| NCT06044844 | Recruiting | Drug: Tofacitinib | Efficacy of Tofacitinib in the Systemic Sclerosis |
Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh |
November 2023 | Phase 2 |
| NCT04424303 | Recruiting | Drug: Tofacitinib | Ulcerative Colitis | Pfizer | December 4, 2020 | |
| NCT06278402 | Completed | Drug: Tofacitinib | Alopecia Areata Alopecia Totalis |
Jinnah Hospital | July 1, 2023 | Phase 3 |
|
CP-690,550 inhibits signal transducer and activator of transcription (STAT)3 nuclear localization in both murine cell lines.Cancer Sci.2008 Jun;99(6):1265-73. |
Effect of CP-690,550 on ex vivo expanded erythroid progenitors.Cancer Sci.2008 Jun;99(6):1265-73. |
CP-690,550-induced poly (ADP-ribose) polymerase (PARP) and caspase-3 cleavage.Cancer Sci.2008 Jun;99(6):1265-73. |
CP-690,550 modulates immunolocalization of signal transducer and activator of transcription (STAT)3.Cancer Sci.2008 Jun;99(6):1265-73. |
 |