
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
VX-11e is a novel, potent, selective, and orally bioavailable ERK2 (extracellular signal-related kinase 2) inhibitor with potential anticancer activity. With a Ki of<2 which='' is=''>200-fold more selective than other kinases, it inhibits ERK2 in cells. Extracellular signal-regulated kinases, or ERK, are an essential part of the Ras/Raf/MEK/ERK signal transduction pathway, an oncogenic pathway linked to a number of human cancers. The oral bioavailability of VX-11e in rats and mice is good. VX-11e (50 mg/kg, p.o.) significantly inhibits pRSK activity and slows the growth of human melanoma RPDX tumors in NSG mice. VX-11e significantly improves tumor growth inhibition when used in conjunction with BKM120.
| Targets |
ERK2 (Ki = 2 nM); GSK3 (Ki = 395 ); AURA (Ki = 540 ); CDK2 (Ki = 852 )
|
|---|---|
| ln Vitro |
VX-11e has an IC50 of 48 nM and potently inhibits cell proliferation in HT29 cells.
|
| ln Vivo |
VX-11e exhibits good oral bioavailability in both rats and mice.[1] VX-11e (50 mg/kg, p.o.) causes a robust inhibition of pRSK and slows the growth of tumors in NSG mice carrying human melanoma RPDX tumors. VX-11e significantly enhances the inhibition of tumor growth when combined with BKM120.[2]
|
| Enzyme Assay |
Compounds are assayed for the inhibition of ERK2 by a spectophotometric coupled-enzyme assay. In this assay, a fixed concentration of activated ERK2 (10 nM) is incubated with various concentrations of the compounds in DMSO (2.5%) for 10 min. at 30°C in 0.1 mol/L HEPES buffer, pH=7.5, containing 10 mM MgCl2, 2.5 mM phosphoenolpyruvate, 200 μM NADH, 150 μg/mL pyruvate kinase, 50 μg/mL lactate dehydrogenase and 200 μM erktide peptide. The addition of 65 μM ATP starts the reaction. Monitoring is done of the absorbance loss at 340 nM.
|
| Cell Assay |
The incorporation of 3H-thymidine is used to gauge cell proliferation. In a 96-well plate using growth medium, RPMI 1640 containing 10% FBS, the cells are plated at a density of 10,000 cells per well. Compounds are added in successively diluted amounts. 48 hours at 37°C are spent incubating the cells and compounds. 0.4 Ci of 3H-thymidine is added to each well for 8 hours after 48 hours, and then the wells are put back into the incubator at 37°C. The Wallac 1205 BETAPLATE liquid scintillation counter is used to calculate the CPM after the cells have been harvested using a Tomtec 96-well cell harvester.
|
| Animal Protocol |
Prior to the therapy experiments, NSG mice are used to expand in vivo human melanoma RPDX tumors. 50 NSG mice (1:10) are implanted with a collection of tumor fragments that were banked from early mouse passages. These tumors are removed once they have reached the protocol's maximum permitted volume (1,000 mm3), digested, and stored as live cells. To be used in the therapy experiments, a smaller portion of this stock is implanted at a 1:5 ratio into NSG mice while the larger portion is kept as a master bank. With PLX4720 200 ppm chemical additive diet at approximately clinical plasma levels, the expansion phase is continuously under drug pressure. The plasma concentrations of PLX4720 (103.7 μg/mL ±3.2 after 7 days) are comparable to the steady-state concentrations observed in patients receiving vemurafenib 960 mg twice daily (130.6 μg/mL±71.78). Animals are randomly assigned to treatment groups when tumors measure 200 mm3 by caliper measurement, and then there is a 3-day ishout phase. Two times per week, caliper measurements are used to determine tumor size. After two weeks of treatment or as needed for animal welfare, mice are sacrificed. When tumor control is achieved as indicated, dosing is extended. In order to extract proteins, tumor tissue is snap-frozen in liquid N2 and preserved in formalin (for FFPE). 4 hours after the final dose, the treatment groups are sacrificed.
|
| References | |
| Additional Infomation |
4-[2-(2-chloro-4-fluoroanilino)-5-methyl-4-pyrimidinyl]-N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-1H-pyrrole-2-carboxamide is a heteroarene and an aromatic amide.
|
| Molecular Formula |
C24H20CL2FN5O2
|
|
|---|---|---|
| Molecular Weight |
500.35
|
|
| Exact Mass |
499.097
|
|
| Elemental Analysis |
C, 57.61; H, 4.03; Cl, 14.17; F, 3.80; N, 14.00; O, 6.40
|
|
| CAS # |
896720-20-0
|
|
| Related CAS # |
(R)-VX-11e;1680187-43-2
|
|
| PubChem CID |
11634725
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Index of Refraction |
1.674
|
|
| LogP |
5.09
|
|
| Hydrogen Bond Donor Count |
4
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
7
|
|
| Heavy Atom Count |
34
|
|
| Complexity |
679
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
CC1C=NC(NC2C=CC(F)=CC=2Cl)=NC=1C1=CNC(C(=O)N[C@@H](C2C=CC=C(Cl)C=2)CO)=C1
|
|
| InChi Key |
WUTVMXLIGHTZJC-OAQYLSRUSA-N
|
|
| InChi Code |
InChI=1S/C24H20Cl2FN5O2/c1-13-10-29-24(31-19-6-5-17(27)9-18(19)26)32-22(13)15-8-20(28-11-15)23(34)30-21(12-33)14-3-2-4-16(25)7-14/h2-11,21,28,33H,12H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1
|
|
| Chemical Name |
4-[2-(2-chloro-4-fluoroanilino)-5-methylpyrimidin-4-yl]-N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-1H-pyrrole-2-carboxamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3.25 mg/mL (6.50 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3.25 mg/mL (6.50 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9986 mL | 9.9930 mL | 19.9860 mL | |
| 5 mM | 0.3997 mL | 1.9986 mL | 3.9972 mL | |
| 10 mM | 0.1999 mL | 0.9993 mL | 1.9986 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
|---|
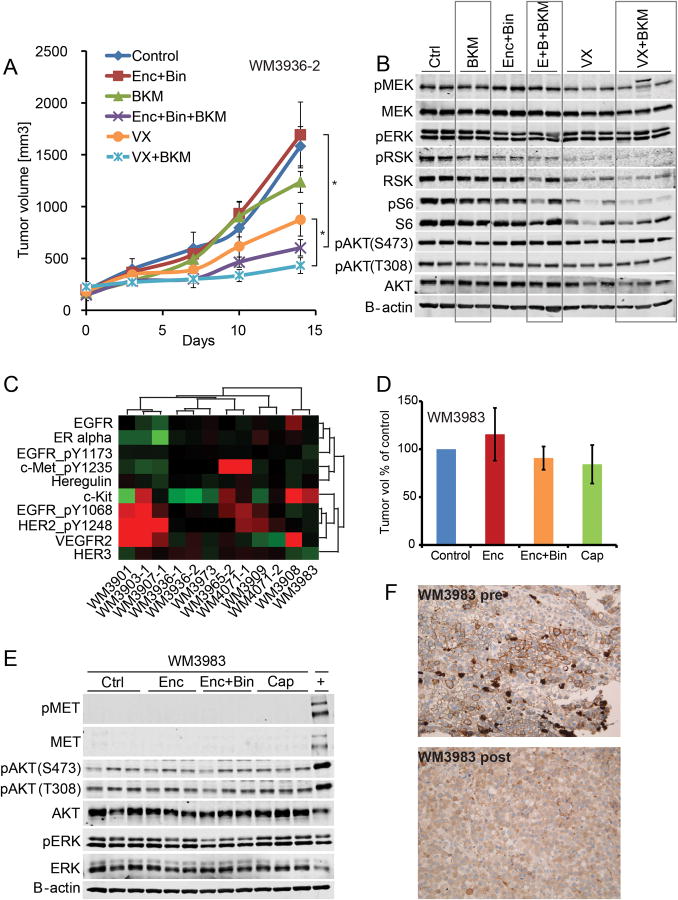 dual pathway inhibition controls tumor growth.Clin Cancer Res.2016 Apr 1;22(7):1592-602. |